IL-13-induced proliferation of airway epithelial cells: mediation by intracellular growth factor mobilization and ADAM17
- PMID: 17620132
- PMCID: PMC1976612
- DOI: 10.1186/1465-9921-8-51
IL-13-induced proliferation of airway epithelial cells: mediation by intracellular growth factor mobilization and ADAM17
Abstract
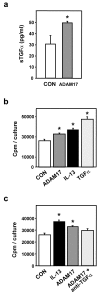
Background: The pleiotrophic cytokine interleukin (IL)-13 features prominently in allergic and inflammatory diseases. In allergic asthma, IL-13 is well established as an inducer of airway inflammation and tissue remodeling. We demonstrated previously that IL-13 induces release of transforming growth factor-alpha (TGFalpha) from human bronchial epithelial cells, with proliferation of these cells mediated by the autocrine/paracrine action of this growth factor. TGFalpha exists as an integral membrane protein and requires proteolytic processing to its mature form, with a disintegrin and metalloproteinase (ADAM)17 responsible for this processing in a variety of tissues.
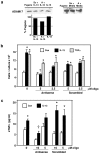
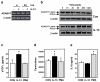
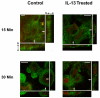
Methods: In this study, normal human bronchial epithelial (NHBE) cells grown in air/liquid interface (ALI) culture were used to examine the mechanisms whereby IL-13 induces release of TGFalpha and cellular proliferation. Inhibitors and antisense RNA were used to examine the role of ADAM17 in these processes, while IL-13-induced changes in the intracellular expression of TGFalpha and ADAM17 were visualized by confocal microscopy.
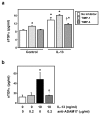
Results: IL-13 was found to induce proliferation of NHBE cells, and release of TGFalpha, in an ADAM17-dependent manner; however, this IL-13-induced proliferation did not appear to result solely from ADAM17 activation. Rather, IL-13 induced a change in the location of TGFalpha expression from intracellular to apical regions of the NHBE cells. The apical region was also found to be a site of significant ADAM17 expression, even prior to IL-13 stimulation.
Conclusion: Results from this study indicate that ADAM17 mediates IL-13-induced proliferation and TGFalpha shedding in NHBE cells. Furthermore, they provide the first example wherein a cytokine (IL-13) induces a change in the intracellular expression pattern of a growth factor, apparently inducing redistribution of intracellular stores of TGFalpha to the apical region of NHBE cells where expression of ADAM17 is prominent. Thus, IL-13-induced, ADAM17-mediated release of TGFalpha, and subsequent epithelial cell proliferation, could contribute to the epithelial hypertrophy, as well as other features, associated with airway remodeling in allergic asthma.
Figures

References
-
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. - PubMed
-
- Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–G499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

