ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity
- PMID: 17620134
- PMCID: PMC1939850
- DOI: 10.1186/1750-1326-2-14
ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity
Abstract
Background: The generation of the amyloid-beta peptide (Abeta) through the proteolytic processing of the amyloid precursor protein (APP) is a central event in the pathogenesis of Alzheimer's disease (AD). Recent studies highlight APP endocytosis and localization to lipid rafts as important events favoring amyloidogenic processing. However, the precise mechanisms underlying these events are poorly understood. ApoER2 is a member of the low density lipoprotein receptor (LDL-R) family exhibiting slow endocytosis rate and a significant association with lipid rafts. Despite the important neurophysiological roles described for ApoER2, little is known regarding how ApoER2 regulates APP trafficking and processing.
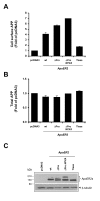
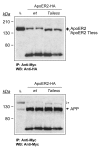
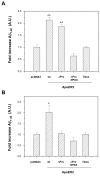
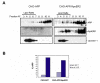
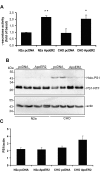
Results: Here, we demonstrate that ApoER2 physically interacts and co-localizes with APP. Remarkably, we found that ApoER2 increases cell surface APP levels and APP association with lipid rafts. The increase of cell surface APP requires the presence of ApoER2 cytoplasmic domain and is a result of decreased APP internalization rate. Unexpectedly, ApoER2 expression correlated with a significant increase in Abeta production and reduced levels of APP-CTFs. The increased Abeta production was dependent on the integrity of the NPxY endocytosis motif of ApoER2. We also found that expression of ApoER2 increased APP association with lipid rafts and increased gamma-secretase activity, both of which might contribute to increased Abeta production.
Conclusion: These findings show that ApoER2 negatively affects APP internalization. However, ApoER2 expression stimulates Abeta production by shifting the proportion of APP from the non-rafts to the raft membrane domains, thereby promoting beta-secretase and gamma-secretase mediated amyloidogenic processing and also by incrementing the activity of gamma-secretase.
Figures





References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. - DOI - PMC - PubMed
-
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

