Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury
- PMID: 17620366
- PMCID: PMC1934533
- DOI: 10.2353/ajpath.2007.060805
Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury
Abstract
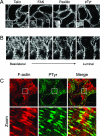
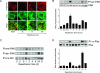
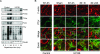
Acute renal failure due to ischemia/reperfusion involves disruption of integrin-mediated cellular adhesion and activation of the extracellular signal-regulated kinase (ERK) pathway. The dynamics of focal adhesion organization and phosphorylation during ischemia/reperfusion in relation to ERK activation are unknown. In control kidneys, protein tyrosine-rich focal adhesions, containing focal adhesion kinase, paxillin, and talin, were present at the basolateral membrane of tubular cells and colocalized with short F-actin stress fibers. Unilateral renal ischemia/reperfusion caused a reversible protein dephosphorylation and loss of focal adhesions. The focal adhesion protein phosphorylation rebounded in a biphasic manner, in association with increased focal adhesion kinase, Src, and paxillin tyrosine phosphorylation. Preceding phosphorylation of these focal adhesion proteins, reperfusion caused increased phosphorylation of ERK. The specific mitogen-activated protein kinase kinase 1/2 inhibitor U0126 prevented ERK activation and attenuated focal adhesion kinase, paxillin, and Src phosphorylation, focal adhesion restructuring, and ischemia/reperfusion-induced renal injury. We propose a model whereby ERK activation enhanced protein tyrosine phosphorylation during ischemia/reperfusion, thereby driving the dynamic dissolution and restructuring of focal adhesions and F-actin cytoskeleton during reperfusion and renal injury.
Figures


References
-
- Molina A, Ubeda M, Escribese MM, Garcia-Bermejo L, Sancho D, de Lema GP, Liano F, Cabanas C, Sanchez-Madrid F, Mampaso F. Renal ischemia/reperfusion injury: functional tissue preservation by anti-activated β1 integrin therapy. J Am Soc Nephrol. 2005;16:374–382. - PubMed
-
- Zalewska T, Makarewicz D, Janik B, Ziemka-Nalecz M. Neonatal cerebral hypoxia-ischemia: involvement of FAK-dependent pathway. Int J Dev Neurosci. 2005;23:657–662. - PubMed
-
- Zalewska T, Ziemka-Nalecz M, Domanska-Janik K. Transient forebrain ischemia effects interaction of Src FAK, and PYK2 with the NR2B subunit of N-methyl-D-aspartate receptor in gerbil hippocampus. Brain Res. 2005;1042:214–223. - PubMed
-
- Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol. 1998;275:C711–C731. - PubMed
-
- Raman N, Atkinson SJ. Rho controls actin cytoskeletal assembly in renal epithelial cells during ATP depletion and recovery. Am J Physiol. 1999;276:C1312–C1324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

