UXT is a novel and essential cofactor in the NF-kappaB transcriptional enhanceosome
- PMID: 17620405
- PMCID: PMC2064443
- DOI: 10.1083/jcb.200611081
UXT is a novel and essential cofactor in the NF-kappaB transcriptional enhanceosome
Abstract
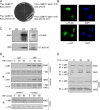
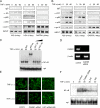
As a latent transcription factor, nuclear factor kappaB (NF-kappaB) translocates from the cytoplasm into the nucleus upon stimulation and mediates the expression of genes that are important in immunity, inflammation, and development. However, little is known about how it is regulated inside the nucleus. By a two-hybrid approach, we identify a prefoldin-like protein, ubiquitously expressed transcript (UXT), that is expressed predominantly and interacts specifically with NF-kappaB inside the nucleus. RNA interference knockdown of UXT leads to impaired NF-kappaB activity and dramatically attenuates the expression of NF-kappaB-dependent genes. This interference also sensitizes cells to apoptosis by tumor necrosis factor-alpha. Furthermore, UXT forms a dynamic complex with NF-kappaB and is recruited to the NF-kappaB enhanceosome upon stimulation. Interestingly, the UXT protein level correlates with constitutive NF-kappaB activity in human prostate cancer cell lines. The presence of NF-kappaB within the nucleus of stimulated or constitutively active cells is considerably diminished with decreased endogenous UXT levels. Our results reveal that UXT is an integral component of the NF-kappaB enhanceosome and is essential for its nuclear function, which uncovers a new mechanism of NF-kappaB regulation.
Figures







References
-
- Barkett, M., and T.D. Gilmore. 1999. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 18:6910–6924. - PubMed
-
- Chan, H.M., and N.B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363–2373. - PubMed
-
- Chen, F.E., D.B. Huang, Y.Q. Chen, and G. Ghosh. 1998. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 391:410–413. - PubMed
-
- Chen, L.F., and W.C. Greene. 2004. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 5:392–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

