The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor
- PMID: 17620406
- PMCID: PMC2064440
- DOI: 10.1083/jcb.200612046
The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor
Abstract
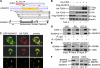
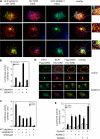
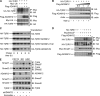
Transforming growth factor-beta (TGF-beta) regulates a wide variety of biological processes through two types of Ser/Thr transmembrane receptors: the TGF-beta type I receptor and the TGF-beta type II receptor (TbetaRII). Upon ligand binding, TGF-beta type I receptor activated by TbetaRII propagates signals to Smad proteins, which mediate the activation of TGF-beta target genes. In this study, we identify ADAM12 (a disintegrin and metalloproteinase 12) as a component of the TGF-beta signaling pathway that acts through association with TbetaRII. We found that ADAM12 functions by a mechanism independent of its protease activity to facilitate the activation of TGF-beta signaling, including the phosphorylation of Smad2, association of Smad2 with Smad4, and transcriptional activation. Furthermore, ADAM12 induces the accumulation of TbetaRII in early endosomal vesicles and stabilizes the TbetaRII protein presumably by suppressing the association of TbetaRII with Smad7. These results define ADAM12 as a new partner of TbetaRII that facilitates its trafficking to early endosomes in which activation of the Smad pathway is initiated.
Figures

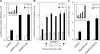
References
-
- Derynck, R., R.J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29:117–129. - PubMed
-
- Di Guglielmo, G.M., C. Le Roy, A.F. Goodfellow, and J.L. Wrana. 2003. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5:410–421. - PubMed
-
- Duffy, M.J., D.J. Lynn, A.T. Lloyd, and C.M. O'Shea. 2003. The ADAMs family of proteins: from basic studies to potential clinical applications. Thromb. Haemost. 89:622–631. - PubMed
-
- Dumont, E., F. Lallemand, C. Prunier, N. Ferrand, A. Guillouzo, B. Clement, A. Atfi, and N. Theret. 2003. Evidence for a role of Smad3 and Smad2 in stabilization of the tumor-derived mutant Smad2.Q407R. J. Biol. Chem. 278:24881–24887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

