Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation
- PMID: 17620407
- PMCID: PMC2064439
- DOI: 10.1083/jcb.200612071
Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation
Abstract
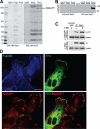
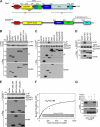
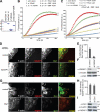
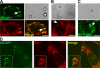
The Diaphanous-related formin Dia1 nucleates actin polymerization, thereby regulating cell shape and motility. Mechanisms that control the cellular location of Dia1 to spatially define actin polymerization are largely unknown. In this study, we identify the cytoskeletal scaffold protein IQGAP1 as a Dia1-binding protein that is necessary for its subcellular location. IQGAP1 interacts with Dia1 through a region within the Diaphanous inhibitory domain after the RhoA-mediated release of Dia1 autoinhibition. Both proteins colocalize at the front of migrating cells but also at the actin-rich phagocytic cup in macrophages. We show that IQGAP1 interaction with Dia1 is required for phagocytosis and phagocytic cup formation. Thus, we identify IQGAP1 as a novel component involved in the regulation of phagocytosis by mediating the localization of the actin filament nucleator Dia1.
Figures
References
-
- Brandt, D.T., A. Goerke, M. Heuer, M. Gimona, M. Leitges, E. Kremmer, R. Lammers, H. Haller, and H. Mischak. 2003. Protein kinase C delta induces Src kinase activity via activation of the protein tyrosine phosphatase PTP alpha. J. Biol. Chem. 278:34073–34078. - PubMed
-
- Brown, M.D., and D.B. Sacks. 2006. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 16:242–249. - PubMed
-
- Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 282:1717–1721. - PubMed
-
- Chimini, G., and P. Chavrier. 2000. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2:E191–E196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

