Quaternary structures of tumor suppressor p53 and a specific p53 DNA complex
- PMID: 17620598
- PMCID: PMC1941468
- DOI: 10.1073/pnas.0705069104
Quaternary structures of tumor suppressor p53 and a specific p53 DNA complex
Abstract
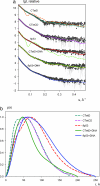
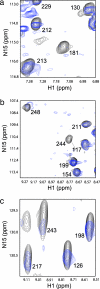
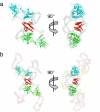
The homotetrameric tumor suppressor p53 consists of folded core and tetramerization domains, linked and flanked by intrinsically disordered segments that impede structure analysis by x-ray crystallography and NMR. Here, we solved the quaternary structure of human p53 in solution by a combination of small-angle x-ray scattering, which defined its shape, and NMR, which identified the core domain interfaces and showed that the folded domains had the same structure in the intact protein as in fragments. We combined the solution data with electron microscopy on immobilized samples that provided medium resolution 3D maps. Ab initio and rigid body modeling of scattering data revealed an elongated cross-shaped structure with a pair of loosely coupled core domain dimers at the ends, which are accessible for binding to DNA and partner proteins. The core domains in that open conformation closed around a specific DNA response element to form a compact complex whose structure was independently determined by electron microscopy. The structure of the DNA complex is consistent with that of the complex of four separate core domains and response element fragments solved by x-ray crystallography and contacts identified by NMR. Electron microscopy on the conformationally mobile, unbound p53 selected a minor compact conformation, which resembled the closed conformation, from the ensemble of predominantly open conformations. A multipronged structural approach could be generally useful for the structural characterization of the rapidly growing number of multidomain proteins with intrinsically disordered regions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
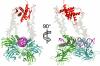
Comment in
-
Quaternary structure of p53: the light at the end of the tunnel.Proc Natl Acad Sci U S A. 2007 Jul 24;104(30):12231-2. doi: 10.1073/pnas.0705319104. Epub 2007 Jul 18. Proc Natl Acad Sci U S A. 2007. PMID: 17640907 Free PMC article. No abstract available.
References
-
- Hainaut P, Wiman KG, editors. 25 Years of p53 Research. New York: Springer; 2005.
-
- Vogelstein B, Lane D, Levine AJ. Nature. 2000;408:307–310. - PubMed
-
- Hupp TR, Lane DP. Cold Spring Harbor Symp Quant Biol. 1994;59:195–206. - PubMed
-
- Hupp TR, Sparks A, Lane DP. Cell. 1995;83:237–245. - PubMed
-
- Dawson R, Muller L, Dehner A, Klein C, Kessler H, Buchner J. J Mol Biol. 2003;332:1131–1141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

