Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis
- PMID: 17620600
- PMCID: PMC1924559
- DOI: 10.1073/pnas.0704756104
Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis
Abstract
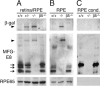
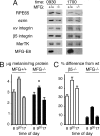
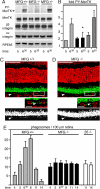
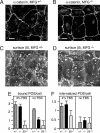
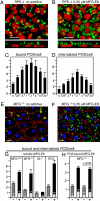
The integrin receptor alphavbeta5 controls two independent forms of interactions of the retinal pigment epithelium (RPE) with adjacent photoreceptor outer segments that are essential for vision. Alphavbeta5 localizes specifically to apical microvilli of the RPE and contributes to retinal adhesion that maintains RPE contacts with intact outer segments at all times. Additionally, alphavbeta5 synchronizes diurnal bursts of RPE phagocytosis that clear photoreceptor outer segment fragments (POS) shed in a circadian rhythm. Dependence of retinal phagocytosis and adhesion on alphavbeta5 receptors suggests that the extracellular matrix ensheathing RPE microvilli contains ligands for this integrin. Here we studied mice lacking expression of functional MFG-E8 to test the contribution of this integrin ligand to alphavbeta5 functions in the retina. Lack of MFG-E8 only minimally reduced retinal adhesion. In contrast, lack of MFG-E8, like lack of alphavbeta5 receptor, eliminated alphavbeta5 downstream signaling involving the engulfment receptor MerTK and peak POS phagocytosis, both of which follow light onset in wild-type retina. MFG-E8-deficient RPE in primary culture retained normal epithelial morphology and levels of apical alphavbeta5 receptors, but showed impaired binding and engulfment of isolated POS. Soluble or POS-bound recombinant MFG-E8 was sufficient to fully restore phagocytosis by MFG-E8-deficient RPE. Furthermore, MFG-E8 supplementation strongly increased POS binding by wild-type and MerTK-deficient RPE, but did not affect POS binding by RPE lacking alphavbeta5. Thus, MFG-E8 stimulates rhythmic POS phagocytosis by ligating apical alphavbeta5 receptors of the RPE. These results identify MFG-E8 as the first extracellular ligand in the retina that is essential for diurnal POS phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin.Biol Cell. 2012 Jun;104(6):326-41. doi: 10.1111/boc.201100076. Epub 2012 Mar 5. Biol Cell. 2012. PMID: 22289110 Free PMC article.
-
Lack of alphavbeta5 integrin receptor or its ligand MFG-E8: distinct effects on retinal function.Ophthalmic Res. 2008;40(3-4):120-3. doi: 10.1159/000119861. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421224 Free PMC article.
-
Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin.J Exp Med. 2004 Dec 20;200(12):1539-45. doi: 10.1084/jem.20041447. Epub 2004 Dec 13. J Exp Med. 2004. PMID: 15596525 Free PMC article.
-
Clearance phagocytosis by the retinal pigment epithelial during photoreceptor outer segment renewal: Molecular mechanisms and relation to retinal inflammation.Immunol Rev. 2023 Oct;319(1):81-99. doi: 10.1111/imr.13264. Epub 2023 Aug 9. Immunol Rev. 2023. PMID: 37555340 Free PMC article. Review.
-
Alphavbeta5 integrin receptors at the apical surface of the RPE: one receptor, two functions.Adv Exp Med Biol. 2008;613:369-75. doi: 10.1007/978-0-387-74904-4_43. Adv Exp Med Biol. 2008. PMID: 18188966 Free PMC article. Review. No abstract available.
Cited by
-
High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C418-36. doi: 10.1152/ajpcell.00001.2016. Epub 2016 Jul 20. Am J Physiol Cell Physiol. 2016. PMID: 27440660 Free PMC article.
-
Fetal hematopoietic stem cells express MFG-E8 during mouse embryogenesis.Exp Mol Med. 2015 Jul 24;47(7):e174. doi: 10.1038/emm.2015.42. Exp Mol Med. 2015. PMID: 26206421 Free PMC article.
-
Regulation of the rhythmic diversity of daily photoreceptor outer segment phagocytosis in vivo.FASEB J. 2022 Oct;36(10):e22556. doi: 10.1096/fj.202200990RR. FASEB J. 2022. PMID: 36165194 Free PMC article. Review.
-
Mfge8 suppresses airway hyperresponsiveness in asthma by regulating smooth muscle contraction.Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):660-5. doi: 10.1073/pnas.1216673110. Epub 2012 Dec 26. Proc Natl Acad Sci U S A. 2013. PMID: 23269839 Free PMC article.
-
A platform for assessing outer segment fate in primary human fetal RPE cultures.Exp Eye Res. 2019 Jan;178:212-222. doi: 10.1016/j.exer.2018.10.008. Epub 2018 Oct 15. Exp Eye Res. 2019. PMID: 30336126 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

