Aristolochic acid and the etiology of endemic (Balkan) nephropathy
- PMID: 17620607
- PMCID: PMC1913550
- DOI: 10.1073/pnas.0701248104
Aristolochic acid and the etiology of endemic (Balkan) nephropathy
Abstract
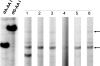
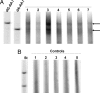
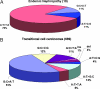
Endemic (Balkan) nephropathy (EN), a devastating renal disease affecting men and women living in rural areas of Bosnia, Bulgaria, Croatia, Romania, and Serbia, is characterized by its insidious onset, invariable progression to chronic renal failure and a strong association with transitional cell (urothelial) carcinoma of the upper urinary tract. Significant epidemiologic features of EN include its focal occurrence in certain villages and a familial, but not inherited, pattern of disease. Our experiments test the hypothesis that chronic dietary poisoning by aristolochic acid is responsible for EN and its associated urothelial cancer. Using (32)P-postlabeling/PAGE and authentic standards, we identified dA-aristolactam (AL) and dG-AL DNA adducts in the renal cortex of patients with EN but not in patients with other chronic renal diseases. In addition, urothelial cancer tissue was obtained from residents of endemic villages with upper urinary tract malignancies. The AmpliChip p53 microarray was then used to sequence exons 2-11 of the p53 gene where we identified 19 base substitutions. Mutations at A:T pairs accounted for 89% of all p53 mutations, with 78% of these being A:T --> T:A transversions. Our experimental results, namely, that (i) DNA adducts derived from aristolochic acid (AA) are present in renal tissues of patients with documented EN, (ii) these adducts can be detected in transitional cell cancers, and (iii) A:T --> T:A transversions dominate the p53 mutational spectrum in the upper urinary tract malignancies found in this population lead to the conclusion that dietary exposure to AA is a significant risk factor for EN and its attendant transitional cell cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Djukanović L, Radovanović Z. In: Clinical Nephrotoxins. De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Dordrecht, The Netherlands: Kluwer Academic; 2003. pp. 587–601.
-
- Stefanović V, Cosyns JP. In: Oxford Textbook of Clinical Nephrology. Davison AM, Cameron JS, Grunfeld JP, Ponticelli C, editors. New York: Oxford Univ Press; 2005. pp. 1095–101.
-
- Tancev I, Evstatijev P, Dorosiev D, Penceva Z, Cvetkov G. Savr Med. 1956;7:14–29. - PubMed
-
- Pichler O, Bobinac E, Miljuš B, Sindik A. Liječ Vjesn. 1959;81:295–306.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

