Electrochemical attosyringe
- PMID: 17620612
- PMCID: PMC1924598
- DOI: 10.1073/pnas.0705102104
Electrochemical attosyringe
Abstract
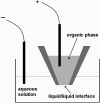
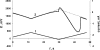
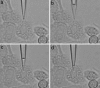
The ability to manipulate ultrasmall volumes of liquids is essential in such diverse fields as cell biology, microfluidics, capillary chromatography, and nanolithography. In cell biology, it is often necessary to inject material of high molecular weight (e.g., DNA, proteins) into living cells because their membranes are impermeable to such molecules. All techniques currently used for microinjection are plagued by two common problems: the relatively large injector size and volume of injected fluid, and poor control of the amount of injected material. Here we demonstrate the possibility of electrochemical control of the fluid motion that allows one to sample and dispense attoliter-to-picoliter (10(-18) to 10(-12) liter) volumes of either aqueous or nonaqueous solutions. By changing the voltage applied across the liquid/liquid interface, one can produce a sufficient force to draw solution inside a nanopipette and then inject it into an immobilized biological cell. A high success rate was achieved in injections of fluorescent dyes into cultured human breast cells. The injection of femtoliter-range volumes can be monitored by video microscopy, and current/resistance-based approaches can be used to control injections from very small pipettes. Other potential applications of the electrochemical syringe include fluid dispensing in nanolithography and pumping in microfluidic systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Matsuoka H, Komazaki T, Mukai Y, Shibusawa M, Akane H, Chaki A, Uetake N, Saito M. J Biotechnol. 2005;116:185–194. - PubMed
-
- Neuman E, Sowers AE, Jordan CA, editors. Electroporation and Electrofusion in Cell Biology. New York: Plenum; 1989.
-
- Knoblauch M, van Bel AJE. Plant Cell. 1998;10:35–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

