Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA
- PMID: 17620619
- PMCID: PMC1924560
- DOI: 10.1073/pnas.0705245104
Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA
Abstract
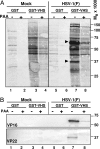
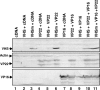
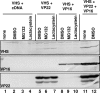
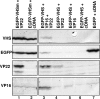
The virion host shutoff (vhs) protein encoded by the U(L)41 gene of herpes simplex virus 1 is an endoribonuclease. The enzyme is introduced into the cell during unpackaging of the virion upon entry and selectively degrades mRNA for several hours. The RNase activity ceases after the onset of synthesis of late (gamma) viral proteins. Here we report that vhs protein does not accumulate in cells transiently transfected with only a plasmid encoding the U(L)41 gene. However, vhs does accumulate in cells cotransfected with plasmids expressing two other tegument proteins, VP16 and VP22. vhs does not directly interact with VP22 but, instead, binds VP22 only in the presence of VP16. In contrast to these findings, the amounts of vhs mRNA accumulating in the cells transfected solely with vhs are not significantly different from those detected in cells coexpressing vhs, VP16, and VP22. We conclude from these studies that the steady state of vhs mRNA, reflecting synthesis and turnover of mRNA, is not affected by the interaction of vhs protein with VP16 with VP22. A model is proposed in which the vhs protein may function to sequester mRNAs in compartments inaccessible to the cellular translational machinery and that VP16 and VP22 rescue the mRNAs by interacting with the vhs protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Multiple Posttranscriptional Strategies To Regulate the Herpes Simplex Virus 1 vhs Endoribonuclease.J Virol. 2018 Aug 16;92(17):e00818-18. doi: 10.1128/JVI.00818-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925667 Free PMC article.
-
Selective ablation of virion host shutoff protein RNase activity attenuates herpes simplex virus 2 in mice.J Virol. 2008 Apr;82(7):3642-53. doi: 10.1128/JVI.02409-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234805 Free PMC article.
-
Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function.EMBO J. 1996 May 15;15(10):2575-81. EMBO J. 1996. PMID: 8665865 Free PMC article.
-
Virus-encoded endonucleases: expected and novel functions.Wiley Interdiscip Rev RNA. 2013 Nov-Dec;4(6):693-708. doi: 10.1002/wrna.1188. Epub 2013 Jul 30. Wiley Interdiscip Rev RNA. 2013. PMID: 23900973 Review.
-
Early shutoff of host protein synthesis in cells infected with herpes simplex viruses.Acta Virol. 2001;45(5-6):269-77. Acta Virol. 2001. PMID: 12083325 Review.
Cited by
-
Gammaherpesviral gene expression and virion composition are broadly controlled by accelerated mRNA degradation.PLoS Pathog. 2014 Jan;10(1):e1003882. doi: 10.1371/journal.ppat.1003882. Epub 2014 Jan 16. PLoS Pathog. 2014. PMID: 24453974 Free PMC article.
-
Role of the DNA Binding Activity of Herpes Simplex Virus 1 VP22 in Evading AIM2-Dependent Inflammasome Activation Induced by the Virus.J Virol. 2021 Mar 1;95(5):e02172-20. doi: 10.1128/JVI.02172-20. Epub 2020 Dec 9. J Virol. 2021. PMID: 33298538 Free PMC article.
-
Tristetraprolin Recruits the Herpes Simplex Virion Host Shutoff RNase to AU-Rich Elements in Stress Response mRNAs To Enable Their Cleavage.J Virol. 2015 May;89(10):5643-50. doi: 10.1128/JVI.00091-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762736 Free PMC article.
-
Duck plague virus UL41 protein inhibits RIG-I/MDA5-mediated duck IFN-β production via mRNA degradation activity.Vet Res. 2022 Mar 18;53(1):22. doi: 10.1186/s13567-022-01043-y. Vet Res. 2022. PMID: 35303942 Free PMC article.
-
The Role of Viral RNA Degrading Factors in Shutoff of Host Gene Expression.Annu Rev Virol. 2022 Sep 29;9(1):213-238. doi: 10.1146/annurev-virology-100120-012345. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671567 Free PMC article. Review.
References
-
- Karr BM, Read GS. Virology. 1999;264:195–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

