Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC ('Arimidex', Tamoxifen alone or in combination) trial
- PMID: 17622238
- PMCID: PMC2360294
- DOI: 10.1038/sj.bjc.6603804
Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC ('Arimidex', Tamoxifen alone or in combination) trial
Abstract
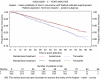
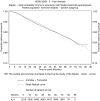
Results from the completed treatment analysis of the ATAC (Arimidex, Tamoxifen alone or in combination) trial indicated that anastrozole was significantly superior to tamoxifen in terms of efficacy and safety in the adjuvant treatment of postmenopausal women with hormone receptor-positive (HR+) early breast cancer. On the basis of these results, this study estimated the cost-effectiveness of anastrozole vs tamoxifen, from the perspective of the UK National Health Service (NHS). A Markov model was developed using the 5-year completed treatment analysis from the ATAC trial (ISRCTN18233230), as well as data obtained from published literature and expert opinion. Resource utilisation data and associated costs (2003-4 UK pound) were compiled from standard sources and expert opinion. Utility scores for a number of health states were obtained from a cross-sectional study of 26 representative patients using the standard gamble technique. The utility scores were then inserted into the model to obtain cost per quality adjusted life-year (QALY) gained. Costs and benefits were discounted at recommended annual rates of the UK Treasury (3.5%). Modelled for 25 years, anastrozole, relative to generic tamoxifen, was estimated to result in 0.244 QALYs gained per patient at an additional cost of pound4315 per patient). The estimated incremental cost-effectiveness of anastrozole compared with tamoxifen was pound17 656 per QALY gained. There was a greater than 90% probability that the cost-effectiveness of anastrozole was below pound30 000 per QALY gained and of the order of 65% that it was below pound20 000 per QALY gained. The results were robust to all parameters tested in sensitivity analysis. Compared with commonly accepted thresholds, anastrozole is a cost-effective alternative to generic tamoxifen in adjuvant treatment of postmenopausal women with HR+ early breast cancer from the UK NHS perspective.
Figures


Similar articles
-
Cost-effectiveness analysis of anastrozole versus tamoxifen as primary adjuvant therapy for postmenopausal women with early breast cancer: a US healthcare system perspective. The 5-year completed treatment analysis of the ATAC ('Arimidex', Tamoxifen Alone or in Combination) trial.Breast Cancer Res Treat. 2007 Dec;106(2):229-38. doi: 10.1007/s10549-006-9483-6. Epub 2007 Jan 24. Breast Cancer Res Treat. 2007. PMID: 17245540
-
Cost-effectiveness analysis of anastrozole versus tamoxifen in adjuvant therapy for early-stage breast cancer - a health-economic analysis based on the 100-month analysis of the ATAC trial and the German health system.Onkologie. 2010;33(4):155-66. doi: 10.1159/000286233. Epub 2010 Mar 19. Onkologie. 2010. PMID: 20389141 Clinical Trial.
-
Cost-effectiveness of anastrozole, in comparison with tamoxifen, in the adjuvant treatment of early breast cancer in Brazil.Rev Assoc Med Bras (1992). 2009 Jul-Aug;55(4):410-5. doi: 10.1590/s0104-42302009000400015. Rev Assoc Med Bras (1992). 2009. PMID: 19750307
-
Letrozole: a pharmacoeconomic review of its use in postmenopausal women with breast cancer.Pharmacoeconomics. 2006;24(5):495-517. doi: 10.2165/00019053-200624050-00007. Pharmacoeconomics. 2006. PMID: 16706574 Review.
-
Three years' follow-up from the ATAC trial is sufficient to change clinical practice: a debate.Breast Cancer Res Treat. 2003;80 Suppl 1:S3-11; discussion S13-8. doi: 10.1023/a:1025455130476. Breast Cancer Res Treat. 2003. PMID: 14535530 Review.
Cited by
-
Anastrozole: a review of its use in postmenopausal women with early-stage breast cancer.Drugs. 2008;68(9):1319-40. doi: 10.2165/00003495-200868090-00007. Drugs. 2008. PMID: 18547136 Review.
-
Gynaecological cancer surveillance for women with Lynch syndrome: systematic review and cost-effectiveness evaluation.Health Technol Assess. 2024 Aug;28(41):1-228. doi: 10.3310/VBXX6307. Health Technol Assess. 2024. PMID: 39246007 Free PMC article.
-
Cost-effectiveness of Tamoxifen, Aromatase Inhibitor, and Switch Therapy (Adjuvant Endocrine Therapy) for Breast Cancer in Hormone Receptor Positive Postmenopausal Women in India.Breast Cancer (Dove Med Press). 2021 Nov 27;13:625-640. doi: 10.2147/BCTT.S331831. eCollection 2021. Breast Cancer (Dove Med Press). 2021. PMID: 34866937 Free PMC article.
-
The total hospital and community UK costs of managing patients with relapsed breast cancer.Br J Cancer. 2009 Feb 24;100(4):598-600. doi: 10.1038/sj.bjc.6604911. Br J Cancer. 2009. PMID: 19223909 Free PMC article.
-
Cost-effectiveness of dual-energy X-ray absorptiometry plus antiresorptive treatment in Australian women with breast cancer who receive aromatase inhibitors.J Bone Miner Metab. 2017 Mar;35(2):199-208. doi: 10.1007/s00774-016-0742-2. Epub 2016 Mar 11. J Bone Miner Metab. 2017. PMID: 26969395
References
-
- ATAC Trialists' Group (2005) Results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365: 60–62 - PubMed
-
- Benedict Á, Christie A (2003) Budget impact analysis of anastrozole as adjuvant therapy in the treatment of early breast cancer in the UK [abstract]. Value Health 6: 735
-
- Briggs A (2001) Handling uncertainty in economic evaluations and presenting the results. In Economic Evaluation in Health Care: Merging Theory with Practice Drummond M, McGuire AM (eds). Oxford University Press, Oxford, UK, pp 172–214
-
- British National Formulary (2003/2004) Available at: http://www.bnf.org/bnf.Accessed March 2003 and Sept 2004
-
- Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A (2006) Quality of life of postmenopausal women in the ATAC (‘Arimidex’ tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Br Cancer Res Treat 100(3): 273–284 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

