Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer
- PMID: 17622246
- PMCID: PMC2360319
- DOI: 10.1038/sj.bjc.6603881
Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer
Abstract
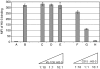
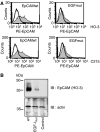
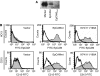
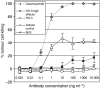
Epithelial cell adhesion molecule EpCAM is a transmembrane glycoprotein that is frequently overexpressed in a variety of carcinomas. This pan-carcinoma antigen has served as the target for a plethora of immunotherapies. Innovative therapeutic approaches include the use of trifunctional antibodies (trAbs) that recruit and activate different types of immune effector cells at the tumour site. The trAb catumaxomab has dual specificity for EpCAM and CD3. In patients with malignant ascites, catumaxomab significantly increased the paracentesis-free interval, corroborating the high efficacy of this therapeutic antibody. Here, we characterised the monoclonal antibody (mAb) HO-3, that is, the EpCAM-binding arm of catumaxomab. Peptide mapping indicated that HO-3 recognises a discontinuous epitope, having three binding sites in the extracellular region of EpCAM. Studies with glycosylation-deficient mutants showed that mAb HO-3 recognised EpCAM independently of its glycosylation status. High-affinity binding was not only detected for mAb HO-3, but also for the monovalent EpCAM-binding arm of catumaxomab with an excellent K(D) of 5.6 x 10(-10) M. Furthermore, trAb catumaxomab was at least a 1000-fold more effective in eliciting the eradication of tumour cells by effector peripheral blood mononuclear cells compared with mAb HO-3. These findings suggest the great therapeutic potential of trAbs and clearly speak in favour of EpCAM-directed cancer immunotherapies.
Figures
References
-
- Armstrong A, Eck SL (2003) EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther 2: 320–326 - PubMed
-
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV (1999) The biology of the 17-1A antigen (Ep-CAM). J Mol Med 77: 699–712 - PubMed
-
- Bjork P, Jonsson U, Svedberg H, Larsson K, Lind P, Dillner J, Hedlund G, Dohlsten M, Kalland T (1993) - Isolation, partial characterization, and molecular cloning of a human colon adenocarcinoma cell-surface glycoprotein recognized by the C215 mouse monoclonal antibody. J Biol Chem 268: 24232–24241 - PubMed
-
- Chong JM, Speicher DW (2001) Determination of disulfide bond assignments and N-glycosylation sites of the human gastrointestinal carcinoma antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J Biol Chem 276: 5804–5813 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

