Apert p.Ser252Trp mutation in FGFR2 alters osteogenic potential and gene expression of cranial periosteal cells
- PMID: 17622301
- PMCID: PMC1952676
- DOI: 10.2119/2007–00027.Fanganiello
Apert p.Ser252Trp mutation in FGFR2 alters osteogenic potential and gene expression of cranial periosteal cells
Abstract
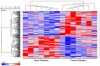
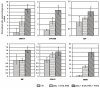
Apert syndrome (AS), a severe form of craniosynostosis, is caused by dominant gain-of-function mutations in FGFR2. Because the periosteum contribution to AS cranial pathophysiology is unknown, we tested the osteogenic potential of AS periosteal cells (p.Ser252Trp mutation) and observed that these cells are more committed toward the osteoblast lineage. To delineate the gene expression profile involved in this abnormal behavior, we performed a global gene expression analysis of coronal suture periosteal cells from seven AS patients (p.Ser252Trp), and matched controls. We identified 263 genes with significantly altered expression in AS samples (118 upregulated, 145 downregulated; SNR >or= |0.4|, P <or= 0.05). Several upregulated genes are involved in positive regulation of cell proliferation and nucleotide metabolism, whereas several downregulated genes are involved in inhibition of cell proliferation, gene expression regulation, cell adhesion, and extracellular matrix organization, and in PIK3-MAPK cascades. AS expression profile was confirmed through real-time PCR of a selected set of genes using RNAs from AS and control cells as well as from control cells treated with high FGF2 concentration, and through the analysis of genes involved in FGF-FGFR signaling. Our results allowed us to: (a) suggest that AS periosteal cells present enhanced osteogenic potential, (b) unravel a specific gene expression signature characteristic of AS periosteal cells which may be associated with their osteogenic commitment, (c) identify a set of novel genes involved in the pathophysiology of AS or other craniosynostotic conditions, and (d) suggest for the first time that the periosteum might be involved in the pathophysiology of AS.
Figures


Similar articles
-
Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome.J Clin Invest. 1998 Mar 15;101(6):1310-7. J Clin Invest. 1998. PMID: 9502772 Free PMC article.
-
The Ser252Trp fibroblast growth factor receptor-2 (FGFR-2) mutation induces PKC-independent downregulation of FGFR-2 associated with premature calvaria osteoblast differentiation.Exp Cell Res. 2000 Apr 10;256(1):158-67. doi: 10.1006/excr.2000.4820. Exp Cell Res. 2000. PMID: 10739663
-
Increased expression of protein kinase Calpha, interleukin-1alpha, and RhoA guanosine 5'-triphosphatase in osteoblasts expressing the Ser252Trp fibroblast growth factor 2 receptor Apert mutation: identification by analysis of complementary DNA microarray.J Bone Miner Res. 2001 Apr;16(4):705-12. doi: 10.1359/jbmr.2001.16.4.705. J Bone Miner Res. 2001. PMID: 11315998
-
Clinicogenetic study of Turkish patients with syndromic craniosynostosis and literature review.Pediatr Neurol. 2014 May;50(5):482-90. doi: 10.1016/j.pediatrneurol.2014.01.023. Epub 2014 Jan 11. Pediatr Neurol. 2014. PMID: 24656465 Review.
-
Apert and Crouzon syndromes: clinical findings, genes and extracellular matrix.J Craniofac Surg. 2005 May;16(3):361-8. doi: 10.1097/01.scs.0000157078.53871.11. J Craniofac Surg. 2005. PMID: 15915098 Review.
Cited by
-
Understanding craniosynostosis as a growth disorder.Wiley Interdiscip Rev Dev Biol. 2016 Jul;5(4):429-59. doi: 10.1002/wdev.227. Epub 2016 Mar 22. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27002187 Free PMC article. Review.
-
Research advances in Apert syndrome.J Oral Biol Craniofac Res. 2018 Sep-Dec;8(3):194-199. doi: 10.1016/j.jobcr.2017.05.006. Epub 2017 May 25. J Oral Biol Craniofac Res. 2018. PMID: 30191107 Free PMC article. Review.
-
Stem cell researches in Brazil: present and future challenges.Stem Cell Rev Rep. 2009 Jun;5(2):123-9. doi: 10.1007/s12015-009-9057-1. Epub 2009 Feb 19. Stem Cell Rev Rep. 2009. PMID: 19225912 Review.
-
Activation of the IGF1 pathway mediates changes in cellular contractility and motility in single-suture craniosynostosis.J Cell Sci. 2016 Feb 1;129(3):483-91. doi: 10.1242/jcs.175976. Epub 2015 Dec 11. J Cell Sci. 2016. PMID: 26659664 Free PMC article.
-
Scalp fibroblasts have a shared expression profile in monogenic craniosynostosis.J Med Genet. 2010 Dec;47(12):803-8. doi: 10.1136/jmg.2009.069617. Epub 2009 Sep 15. J Med Genet. 2010. PMID: 19755431 Free PMC article.
References
-
- Cohen MM, Jr, et al. Birth prevalence study of the Apert syndrome. Am J Med Genet. 1992;42:655–9. - PubMed
-
- Upton J, Zuker RM, editors. Apert syndrome. Vol. 18. WB Saunders; Philadelphia: 1991. Clinics in plastic surgery.
-
- Cohen MM., Jr . Syndromes with craniosynostosis. In: Cohen MM Jr, MacLean RE, editors. Craniosynostosis: Diagnosis, Evaluation, and Management. New York: Oxford University Press; 2000. pp. 309–441.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
