Mild acidosis enhances AMPA receptor-mediated intracellular zinc mobilization in cortical neurons
- PMID: 17622309
- PMCID: PMC1952667
- DOI: 10.2119/2007–00047.Frazzini
Mild acidosis enhances AMPA receptor-mediated intracellular zinc mobilization in cortical neurons
Abstract
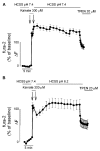
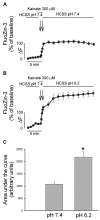
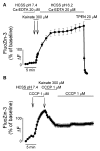
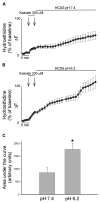
Overactivation of glutamate receptors and subsequent deregulation of the intraneuronal calcium ([Ca2+]i) levels are critical components of the injurious pathways initiated by cerebral ischemia. Another hallmark of stroke is parenchymal acidosis, and we have previously shown that mild acidosis can act as a switch to decrease NMDAR-dependent neuronal loss while potentiating the neuronal loss mediated by AMPARs. Potentiation of AMPAR-mediated neuronal death in an acidotic environment was originally associated only with [Ca2+]i dyshomeostasis, as assessed by Ca2+ imaging; however, intracellular dyshomeostasis of another divalent cation, Zn2+, has recently emerged as another important co-factor in ischemic neuronal injury. Rises in [Zn2+]i greatly contribute to the fluorescent changes of Ca2+-sensitive fluorescent probes, which also have great affinity for Zn2+. We therefore revisited our original findings (Mcdonald et al., 1998) and investigated if AMPAR-mediated fura-2 signals we observed could also be partially due to [Zn2+]i increases. Fura-2 loaded neuronal cultures were exposed to the AMPAR agonist, kainate, in a physiological buffer at pH 7.4 and then washed either at pH 7.4 or pH 6.2. A delayed recovery of fura-2 signals was observed at both pHs. Interestingly this impaired recovery phase was found to be sensitive to chelation of intracellular Zn2+. Experiments with the Zn2+ sensitive (and Ca2+-insensitive) fluorescent probe FluoZin-3 confirmed the idea that AMPAR activation increases [Zn2+]i, a phenomenon that is potentiated by mild acidosis. Additionally, our results show that selective Ca2+ imaging mandates the use of intracellular heavy metal chelators to avoid confounding effects of endogenous metals such as Zn2+.
Figures
Similar articles
-
Acidosis enhances toxicity induced by kainate and zinc exposure in aged cultured astrocytes.Biogerontology. 2006 Oct-Dec;7(5-6):367-74. doi: 10.1007/s10522-006-9051-9. Biogerontology. 2006. PMID: 16964527
-
Kainate-stimulated Zn2+ uptake labels cortical neurons with Ca2+-permeable AMPA/kainate channels.Brain Res. 1998 Jan 19;781(1-2):45-56. doi: 10.1016/s0006-8993(97)01208-0. Brain Res. 1998. PMID: 9507061
-
Acidosis and 5-(N-ethyl-N-isopropyl)amiloride (EIPA) Attenuate Zinc/Kainate Toxicity in Cultured Cerebellar Granule Neurons.Biochemistry (Mosc). 2015 Aug;80(8):1065-72. doi: 10.1134/S000629791508012X. Biochemistry (Mosc). 2015. PMID: 26547075
-
Mercurial-induced alterations in neuronal divalent cation homeostasis.Neurotoxicology. 1996 Spring;17(1):47-61. Neurotoxicology. 1996. PMID: 8784818 Review.
-
Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration.Trends Neurosci. 2000 Aug;23(8):365-71. doi: 10.1016/s0166-2236(00)01610-6. Trends Neurosci. 2000. PMID: 10906800 Review.
Cited by
-
Relationship between Zinc (Zn (2+) ) and Glutamate Receptors in the Processes Underlying Neurodegeneration.Neural Plast. 2015;2015:591563. doi: 10.1155/2015/591563. Epub 2015 May 27. Neural Plast. 2015. PMID: 26106488 Free PMC article. Review.
-
Chelation of hippocampal zinc enhances long-term potentiation and synaptic tagging/capture in CA1 pyramidal neurons of aged rats: implications to aging and memory.Aging Cell. 2017 Feb;16(1):136-148. doi: 10.1111/acel.12537. Epub 2016 Sep 16. Aging Cell. 2017. PMID: 27633878 Free PMC article.
-
Intracellular Zn2+ increases contribute to the progression of excitotoxic Ca2+ increases in apical dendrites of CA1 pyramidal neurons.Neuroscience. 2009 Mar 3;159(1):104-14. doi: 10.1016/j.neuroscience.2008.11.052. Epub 2008 Dec 14. Neuroscience. 2009. PMID: 19135505 Free PMC article.
-
Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin.PLoS One. 2012;7(4):e35482. doi: 10.1371/journal.pone.0035482. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545109 Free PMC article.
-
Acidosis-induced zinc-dependent death of cultured cerebellar granule neurons.Cell Mol Neurobiol. 2010 Aug;30(6):877-83. doi: 10.1007/s10571-010-9516-x. Epub 2010 Apr 7. Cell Mol Neurobiol. 2010. PMID: 20373017 Free PMC article.
References
-
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. - PubMed
-
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. - PubMed
-
- Traynelis S, Cull-Candy S. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–50. - PubMed
-
- Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506:339–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
