Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease
- PMID: 17622324
- PMCID: PMC1952669
- DOI: 10.2119/2007–00036.Maret
Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease
Abstract
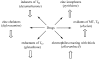
Zinc is involved in virtually all aspects of cellular and molecular biology as a catalytic, structural, and regulatory cofactor in over 1000 proteins. Zinc binding to proteins requires an adequate supply of zinc and intact molecular mechanisms for redistributing zinc ions to make them available at the right time and location. Several dozen gene products participate in this process, in which interactions between zinc and sulfur donors determine the mobility of zinc and establish coupling between cellular redox state and zinc availability. Specifically, the redox properties of metallothionein and its apoprotein thionein are critical for buffering zinc ions and for controlling fluctuations in the range of picomolar concentrations of "free" zinc ions in cellular signaling. Metallothionein and other proteins with sulfur coordination environments are sensitive to redox perturbations and can render cells susceptible to injury when oxidative stress compromises the cellular redox and zinc buffering capacity in chronic diseases. The implications of these fundamental principles for zinc metabolism in type 2 diabetes are briefly discussed.
Figures
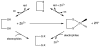
Similar articles
-
The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling.Arch Biochem Biophys. 2007 Jul 15;463(2):188-200. doi: 10.1016/j.abb.2007.02.017. Epub 2007 Mar 7. Arch Biochem Biophys. 2007. PMID: 17391643 Review.
-
Zinc coordination environments in proteins as redox sensors and signal transducers.Antioxid Redox Signal. 2006 Sep-Oct;8(9-10):1419-41. doi: 10.1089/ars.2006.8.1419. Antioxid Redox Signal. 2006. PMID: 16987000 Review.
-
Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc.Exp Gerontol. 2008 May;43(5):363-9. doi: 10.1016/j.exger.2007.11.005. Epub 2007 Nov 28. Exp Gerontol. 2008. PMID: 18171607 Review.
-
Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals.Biometals. 2009 Feb;22(1):149-57. doi: 10.1007/s10534-008-9186-z. Epub 2009 Jan 7. Biometals. 2009. PMID: 19130267
-
Cellular zinc and redox states converge in the metallothionein/thionein pair.J Nutr. 2003 May;133(5 Suppl 1):1460S-2S. doi: 10.1093/jn/133.5.1460S. J Nutr. 2003. PMID: 12730443 Review.
Cited by
-
In silico assessment of S100A12 monomer and dimer structural dynamics: implications for the understanding of its metal-induced conformational changes.J Biol Inorg Chem. 2014 Oct;19(7):1113-20. doi: 10.1007/s00775-014-1149-y. Epub 2014 Jun 19. J Biol Inorg Chem. 2014. PMID: 24944024
-
The genetics of essential metal homeostasis during development.Genesis. 2008 Apr;46(4):214-28. doi: 10.1002/dvg.20382. Genesis. 2008. PMID: 18395838 Free PMC article. Review.
-
Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+.ACS Chem Biol. 2014 Sep 19;9(9):2111-20. doi: 10.1021/cb5004064. Epub 2014 Jul 17. ACS Chem Biol. 2014. PMID: 25011072 Free PMC article.
-
The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis.Antioxidants (Basel). 2023 Oct 31;12(11):1942. doi: 10.3390/antiox12111942. Antioxidants (Basel). 2023. PMID: 38001795 Free PMC article. Review.
-
Ion mobility mass spectrometry and molecular dynamics simulations unravel the conformational stability of zinc metallothionein-2 species.Chem Commun (Camb). 2023 Apr 11;59(30):4471-4474. doi: 10.1039/d2cc06559b. Chem Commun (Camb). 2023. PMID: 36960761 Free PMC article.
References
-
- Hambidge M. Human zinc deficiency. J Nutr. 2000;130:1344S–9S. - PubMed
-
- Sandstead HH, Frederickson CJ, Penland JG. History of zinc related to brain function. J Nutr. 2000;130:496S–502S. - PubMed
-
- Frederickson CJ, Koh J-Y, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. - PubMed
-
- Fong LYY, Jiang YB, Farber JL. Zinc deficiency potentiates induction and progression of lingual and esophageal tumors in p53-deficient mice. Carcinogenesis. 2006;27:1489–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
