Lessons for a national pharmaceuticals strategy in Canada from Australia and New Zealand
- PMID: 17622393
- PMCID: PMC2651914
- DOI: 10.1016/s0828-282x(07)70815-2
Lessons for a national pharmaceuticals strategy in Canada from Australia and New Zealand
Abstract
Background: The provincial formulary review processes in Canada lead to the slow and inequitable availability of new products. In 2004, the exploration of a national pharmaceuticals strategy (NPS) was announced. The pricing policies of New Zealand and Australia have been suggested as possible models for the NPS.
Objective: To compare health care indexes and health care use information from Canada, Australia and New Zealand.
Methods: The 2006 Organisation for Economic Co-operation and Development health data were used to compare health and health care indexes from Canada, Australia and New Zealand between 1994 and 2002 to 2004. The principal focus of the evaluation was cardiovascular and respiratory disorders.
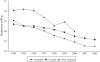
Results: Although the mortality rate from acute myocardial infarction decreased in each country from 1994, it levelled off in New Zealand in 1997, 1998 and 1999. Between 1994 and 2003, the average length of hospital stay for any cause and for cardiovascular disorders was stable in Australia and Canada, but increased in New Zealand, while the rate of hospital discharges for cardiovascular diseases decreased in Canada and Australia, but strongly increased in New Zealand. Over the same period, sales of cardiovascular drugs decreased in New Zealand, while sharply increasing in Canada and Australia.
Conclusions: Although only circumstantial, our results suggest an association between decreasing cardiovascular drug sales and markers of declining cardiovascular health in New Zealand. Careful consideration must be given to the potential consequences of any model for an NPS in Canada, as well as to opportunities provided for discussion and input from health care professionals and patients.
CONTEXTE:: Le processus de révision des listes provinciales de médicaments au Canada conduit à une arrivée lente et inéquitable des nouveaux produits. L’analyse exploratoire d’une stratégie pharmaceutique nationale (SPN) a été annoncée en 2004. On a suggéré d’appliquer les politiques d’établissement des prix en vigueur en Australie et en Nouvelle-Zélande comme modèles possibles de SPN.
BUT:: L’étude avait pour but de comparer les indices de soins de santé et les données sur l’utilisation des soins de santé entre le Canada, l’Australie et la Nouvelle-Zélande.
MÉTHODE:: Nous avons utilisé les données 2006 de l’Organisation de coopération et de développement économiques sur la santé pour comparer les indices de santé et de soins de santé entre le Canada, l’Australie et la Nouvelle-Zélande, de 1994 à 2002 jusqu’en 2004. Le principal point d’intérêt était les maladies respiratoires et cardiovasculaires.
RÉSULTATS:: Même si le taux de mortalité attribuable à l’infarctus du myocarde a diminué dans chacun des pays à partir de 1994, il a atteint un plateau en Nouvelle-Zélande en 1997, 1998 et 1999. La durée moyenne du séjour à l’hôpital, de 1994 à 2003, pour tous types de troubles et pour les troubles cardiovasculaires est restée stable en Australie et au Canada mais a augmenté en Nouvelle-Zélande, tandis que le taux de sortie de l’hôpital pour les maladies cardiovasculaires a diminué au Canada et en Australie mais a considérablement augmenté en Nouvelle-Zélande. Pendant la même période, les ventes de médicaments à action cardiovasculaire ont diminué en Nouvelle-Zélande mais ont fortement augmenté au Canada et en Australie.
CONCLUSIONS:: Bien qu’il ne s’agisse que d’éléments probants indirects, les résultats donnent à penser qu’il existe un lien entre la diminution des ventes de médicaments à action cardiovasculaire et les indicateurs de détérioration de la santé cardiovasculaire en Nouvelle-Zélande. Aussi faudra-t-il porter une attention particulière aux conséquences possibles des différents modèles de SPN au Canada et aux possibilités, pour les professionnels de la santé et pour les patients, de participer aux discussions et d’émettre leurs commentaires.
Figures





Similar articles
-
Leading Causes of Mortality and Prescription Drug Coverage in Canada and New Zealand.Front Public Health. 2020 Oct 30;8:544835. doi: 10.3389/fpubh.2020.544835. eCollection 2020. Front Public Health. 2020. PMID: 33194946 Free PMC article.
-
Would a national pharmaceutical strategy be bad for the cardiovascular health of Canadians?Can J Cardiol. 2007 Jul;23(9):719-20. doi: 10.1016/s0828-282x(07)70816-4. Can J Cardiol. 2007. PMID: 17622394 Free PMC article. No abstract available.
-
Cardiovascular mortality in New Zealand and Australia 1968-1983: how can the diverging trends be explained?N Z Med J. 1986 Jan 22;99(794):1-3. N Z Med J. 1986. PMID: 3456096
-
Rational use of medications: if Canada can't do it ..CMAJ. 2009 Jul 7;181(1-2):15-6. doi: 10.1503/cmaj.090861. CMAJ. 2009. PMID: 19581612 Free PMC article. Review. No abstract available.
-
Disinvestment and Value-Based Purchasing Strategies for Pharmaceuticals: An International Review.Pharmacoeconomics. 2015 Sep;33(9):905-24. doi: 10.1007/s40273-015-0293-8. Pharmacoeconomics. 2015. PMID: 26048353 Review.
Cited by
-
Ontario and New Zealand Pharmaceuticals: Cost and Coverage.Healthc Policy. 2018 May;13(4):23-34. doi: 10.12927/hcpol.2018.25496. Healthc Policy. 2018. PMID: 30052187 Free PMC article.
-
National Pharmacare in Canada: Equality or Equity, Accessibility or Affordability Comment on "Universal Pharmacare in Canada: A Prescription for Equity in Healthcare".Int J Health Policy Manag. 2020 Dec 1;9(12):524-527. doi: 10.15171/ijhpm.2019.146. Int J Health Policy Manag. 2020. PMID: 32610769 Free PMC article.
-
Public-private partnership alternative for a national pharmacare program in Canada.J Pharm Policy Pract. 2023 Feb 6;16(1):21. doi: 10.1186/s40545-023-00526-3. J Pharm Policy Pract. 2023. PMID: 36747233 Free PMC article.
-
The impact of China's national essential medicine system on improving rational drug use in primary health care facilities: an empirical study in four provinces.BMC Health Serv Res. 2014 Oct 25;14:507. doi: 10.1186/s12913-014-0507-3. BMC Health Serv Res. 2014. PMID: 25344413 Free PMC article.
References
-
- Marra CA, Lynd LD, Anis AH, Esdaile JM. Approval process and access to prescription drugs in Canada. Arthritis Rheum. 2006;55:9–11. - PubMed
-
- Rawson NS, Kaitin KI, Thomas KE, Perry G. Drug review in Canada: A comparison with Australia, Sweden, the United Kingdom and the United States. Drug Inf J. 1998;32:1133–41.
-
- Rawson NS. Timeliness of review and approval of new drugs in Canada from 1999 through 2001: Is progress being made? Clin Ther. 2003;25:1230–47. - PubMed
-
- Rawson NSB. Issues in the approval of, access to, and post-marketing follow-up of new drugs in Canada: A personal viewpoint. Pharmacoepidemiol Drug Saf. 2002;11:335–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

