Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase
- PMID: 17622609
- PMCID: PMC2884977
- DOI: 10.1099/vir.0.82940-0
Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase
Abstract
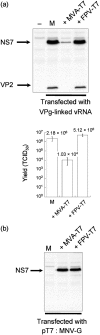
Despite the significant disease burden caused by human norovirus infection, an efficient tissue-culture system for these viruses remains elusive. Murine norovirus (MNV) is an ideal surrogate for the study of norovirus biology, as the virus replicates efficiently in tissue culture and a low-cost animal model is readily available. In this report, a reverse-genetics system for MNV is described, using a fowlpox virus (FWPV) recombinant expressing T7 RNA polymerase to recover genetically defined MNV in tissue culture for the first time. These studies demonstrated that approaches that have proved successful for other members of the family Caliciviridae failed to lead to recovery of MNV. This was due to our observation that vaccinia virus infection had a negative effect on MNV replication. In contrast, FWPV infection had no deleterious effect and allowed the recovery of infectious MNV from cells previously transfected with MNV cDNA constructs. These studies also indicated that the nature of the 3'-terminal nucleotide is critical for efficient virus recovery and that inclusion of a hepatitis delta virus ribozyme at the 3' end can increase the efficiency with which virus is recovered. This system now allows the recovery of genetically defined noroviruses and will facilitate the analysis of the effects of genetic variation on norovirus pathogenesis.
Figures




References
-
- Andrejeva, J., Young, D. F., Goodbourn, S. & Randall, R. E. (2002). Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J Virol 76, 2159–2167. - PMC - PubMed
-
- Boot, H. J., ter Huurne, A. A., Peeters, B. P. & Gielkens, A. L. (1999). Efficient rescue of infectious bursal disease virus from cloned cDNA: evidence for involvement of the 3′-terminal sequence in genome replication. Virology 265, 330–341. - PubMed
-
- Britton, P., Green, P., Kottier, S., Mawditt, K. L., Penzes, Z., Cavanagh, D. & Skinner, M. A. (1996). Expression of bacteriophage T7 RNA polymerase in avian and mammalian cells by a recombinant fowlpox virus. J Gen Virol 77, 963–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

