Effect of branched-chain amino acids on muscle atrophy in cancer cachexia
- PMID: 17623010
- PMCID: PMC2267397
- DOI: 10.1042/BJ20070651
Effect of branched-chain amino acids on muscle atrophy in cancer cachexia
Abstract
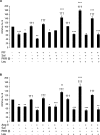
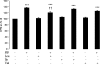
In the present study, the BCAAs (branched-chain amino acids) leucine and valine caused a significant suppression in the loss of body weight in mice bearing a cachexia-inducing tumour (MAC16), producing a significant increase in skeletal muscle wet weight, through an increase in protein synthesis and a decrease in degradation. Leucine attenuated the increased phosphorylation of PKR (double-stranded-RNA-dependent protein kinase) and eIF2alpha (eukaryotic initiation factor 2alpha) in skeletal muscle of mice bearing the MAC16 tumour, due to an increased expression of PP1 (protein phosphatase 1). Weight loss in mice bearing the MAC16 tumour was associated with an increased amount of eIF4E bound to its binding protein 4E-BP1 (eIF4E-binding protein 1), and a progressive decrease in the active eIF4G-eIF4E complex due to hypophosphorylation of 4E-BP1. This may be due to a reduction in the phosphorylation of mTOR (mammalian target of rapamycin), which may also be responsible for the decreased phosphorylation of p70(S6k) (70 kDa ribosomal S6 kinase). There was also a 5-fold increase in the phosphorylation of eEF2 (eukaryotic elongation factor 2), which would also decrease protein synthesis through a decrease in translation elongation. Treatment with leucine increased phosphorylation of mTOR and p70(S6k), caused hyperphosphorylation of 4E-BP1, reduced the amount of 4E-BP1 associated with eIF4E and caused an increase in the eIF4G-eIF4E complex, together with a reduction in phosphorylation of eEF2. These changes would be expected to increase protein synthesis, whereas a reduction in the activation of PKR would be expected to attenuate the increased protein degradation.
Figures
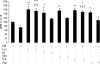





References
-
- Khal J., Hine A. V., Fearon K. C. H., Dejong C. H. C., Tisdale M. J. Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int. J. Biochem. Cell. Biol. 2005;37:2196–2206. - PubMed
-
- Eley H. L., Tisdale M. J. Skeletal muscle atrophy: a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 2007;282:7087–7097. - PubMed
-
- Rowlands A. G., Panniers R., Henshaw E. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J. Biol. Chem. 1988;263:5526–5533. - PubMed
-
- Russell S. T., Eley H., Tisdale M. J. Mechanism of attenuation of angiotensin II-induced protein degradation by insulin-like growth factor I (IGF-I) Cell. Signalling. 2007;19:1797–1806. - PubMed
-
- Norton J. A., Gorschboth C. M., Wesley R. A. Fasting plasma amino acid levels in cancer patients. Cancer. 1985;56:1181–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

