Cloning and expression of a zebrafish SCN1B ortholog and identification of a species-specific splice variant
- PMID: 17623064
- PMCID: PMC1965480
- DOI: 10.1186/1471-2164-8-226
Cloning and expression of a zebrafish SCN1B ortholog and identification of a species-specific splice variant
Abstract
Background: Voltage-gated Na+ channel beta1 (Scn1b) subunits are multi-functional proteins that play roles in current modulation, channel cell surface expression, cell adhesion, cell migration, and neurite outgrowth. We have shown previously that beta1 modulates electrical excitability in vivo using a mouse model. Scn1b null mice exhibit spontaneous seizures and ataxia, slowed action potential conduction, decreased numbers of nodes of Ranvier in myelinated axons, alterations in nodal architecture, and differences in Na+ channel alpha subunit localization. The early death of these mice at postnatal day 19, however, make them a challenging model system to study. As a first step toward development of an alternative model to investigate the physiological roles of beta1 subunits in vivo we cloned two beta1-like subunit cDNAs from D. rerio.
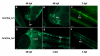
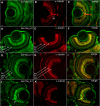
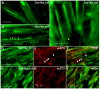
Results: Two beta1-like subunit mRNAs from zebrafish, scn1ba_tv1 and scn1ba_tv2, arise from alternative splicing of scn1ba. The deduced amino acid sequences of Scn1ba_tv1 and Scn1ba_tv2 are identical except for their C-terminal domains. The C-terminus of Scn1ba_tv1 contains a tyrosine residue similar to that found to be critical for ankyrin association and Na+ channel modulation in mammalian beta1. In contrast, Scn1ba_tv2 contains a unique, species-specific C-terminal domain that does not contain a tyrosine. Immunohistochemical analysis shows that, while the expression patterns of Scn1ba_tv1 and Scn1ba_tv2 overlap in some areas of the brain, retina, spinal cord, and skeletal muscle, only Scn1ba_tv1 is expressed in optic nerve where its staining pattern suggests nodal expression. Both scn1ba splice forms modulate Na+ currents expressed by zebrafish scn8aa, resulting in shifts in channel gating mode, increased current amplitude, negative shifts in the voltage dependence of current activation and inactivation, and increases in the rate of recovery from inactivation, similar to the function of mammalian beta1 subunits. In contrast to mammalian beta1, however, neither zebrafish subunit produces a complete shift to the fast gating mode and neither subunit produces complete channel inactivation or recovery from inactivation.
Conclusion: These data add to our understanding of structure-function relationships in Na+ channel beta1 subunits and establish zebrafish as an ideal system in which to determine the contribution of scn1ba to electrical excitability in vivo.
Figures

 and the internal alternate splice acceptor site in exon 5 is indicated by a dashed line and by ▼. The location of stop codons in the resulting mRNAs are indicated. Drawings are not to scale. Consensus splice acceptor sequence [22] and the acceptor sequences found in exon 5 are indicated in the lower portion of the panel. Py: pyrimidine. Pu: purine. Lower case: intronic sequence. Upper case: exonic sequence. The "T" indicated by the red arrow in the internal, alternate acceptor is rare and significantly weakens the site [22]. D. RT-PCR from whole fish RNA demonstrating that both splice variants of scn1ba are expressed in the mRNA pool. The upper band corresponds to scn1ba_tv1 and the lower band corresponds to scn1ba_tv2. Translations of the resulting alternate C-terminal splice products are shown below. The sequence highlighted in green is found in Scn1ba_tv1 and corresponds to the green portion of exon 5 illustrated in panel C. The sequence highlighted in turquoise is found in Scn1ba_tv2 and corresponds to the turquoise portion of exon 5 illustrated in panel C.
and the internal alternate splice acceptor site in exon 5 is indicated by a dashed line and by ▼. The location of stop codons in the resulting mRNAs are indicated. Drawings are not to scale. Consensus splice acceptor sequence [22] and the acceptor sequences found in exon 5 are indicated in the lower portion of the panel. Py: pyrimidine. Pu: purine. Lower case: intronic sequence. Upper case: exonic sequence. The "T" indicated by the red arrow in the internal, alternate acceptor is rare and significantly weakens the site [22]. D. RT-PCR from whole fish RNA demonstrating that both splice variants of scn1ba are expressed in the mRNA pool. The upper band corresponds to scn1ba_tv1 and the lower band corresponds to scn1ba_tv2. Translations of the resulting alternate C-terminal splice products are shown below. The sequence highlighted in green is found in Scn1ba_tv1 and corresponds to the green portion of exon 5 illustrated in panel C. The sequence highlighted in turquoise is found in Scn1ba_tv2 and corresponds to the turquoise portion of exon 5 illustrated in panel C.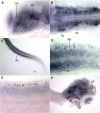






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

