Peripheral nervous system manifestations in a Sandhoff disease mouse model: nerve conduction, myelin structure, lipid analysis
- PMID: 17623103
- PMCID: PMC1976615
- DOI: 10.1186/1477-5751-6-8
Peripheral nervous system manifestations in a Sandhoff disease mouse model: nerve conduction, myelin structure, lipid analysis
Abstract
Background: Sandhoff disease is an inherited lysosomal storage disease caused by a mutation in the gene for the beta-subunit (Hexb gene) of beta-hexosaminidase A (alphabeta) and B (beta beta). The beta-subunit together with the GM2 activator protein catabolize ganglioside GM2. This enzyme deficiency results in GM2 accumulation primarily in the central nervous system. To investigate how abnormal GM2 catabolism affects the peripheral nervous system in a mouse model of Sandhoff disease (Hexb-/-), we examined the electrophysiology of dissected sciatic nerves, structure of central and peripheral myelin, and lipid composition of the peripheral nervous system.
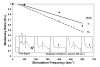
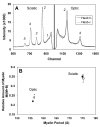
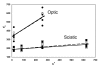
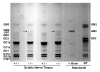
Results: We detected no significant difference in signal impulse conduction velocity or any consistent change in the frequency-dependent conduction slowing and failure between freshly dissected sciatic nerves from the Hexb+/- and Hexb-/- mice. The low-angle x-ray diffraction patterns from freshly dissected sciatic and optic nerves of Hexb+/- and Hexb-/- mice showed normal myelin periods; however, Hexb-/- mice displayed a approximately 10% decrease in the relative amount of compact optic nerve myelin, which is consistent with the previously established reduction in myelin-enriched lipids (cerebrosides and sulfatides) in brains of Hexb-/- mice. Finally, analysis of lipid composition revealed that GM2 content was present in the sciatic nerve of the Hexb-/- mice (undetectable in Hexb+/-).
Conclusion: Our findings demonstrate the absence of significant functional, structural, or compositional abnormalities in the peripheral nervous system of the murine model for Sandhoff disease, but do show the potential value of integrating multiple techniques to evaluate myelin structure and function in nervous system disorders.
Figures
References
-
- Taylor C, Marta C, Bansal R, Pfeiffer S. The transport, assembly, and function of myelin lipids. In: Lazzarini RA, Griffin JW, Lassmann H, Nave K-A, Miller RH, Trapp BD, editor. Myelin Biology and Disorders 1. Vol. 1. Amsterdam: Elsevier/Academic Press; 2004. pp. 57–88.
-
- Gravel RA, Kaback MM, Proia RL, Sandhoff K, Suzuki K, Suzuki K. The GM2 gangliosidoses. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editor. The Metabolic and Molecular Bases of Inherited Disease. Vol. 1. New York: McGraw-Hill; 1995. pp. 3827–3876.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

