Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids
- PMID: 17623659
- PMCID: PMC3119455
- DOI: 10.1074/jbc.M702810200
Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids
Abstract
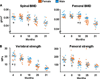
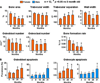
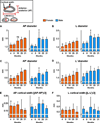
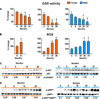
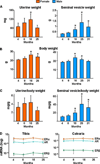
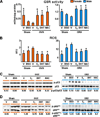
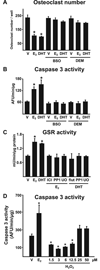
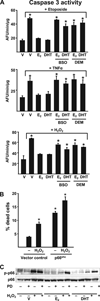
Both aging and loss of sex steroids have adverse effects on skeletal homeostasis, but whether and how they may influence each others negative impact on bone remains unknown. We report herein that both female and male C57BL/6 mice progressively lost strength (as determined by load-to-failure measurements) and bone mineral density in the spine and femur between the ages of 4 and 31 months. These changes were temporally associated with decreased rate of remodeling as evidenced by decreased osteoblast and osteoclast numbers and decreased bone formation rate; as well as increased osteoblast and osteocyte apoptosis, increased reactive oxygen species levels, and decreased glutathione reductase activity and a corresponding increase in the phosphorylation of p53 and p66(shc), two key components of a signaling cascade that are activated by reactive oxygen species and influences apoptosis and lifespan. Exactly the same changes in oxidative stress were acutely reproduced by gonadectomy in 5-month-old females or males and reversed by estrogens or androgens in vivo as well as in vitro. We conclude that the oxidative stress that underlies physiologic organismal aging in mice may be a pivotal pathogenetic mechanism of the age-related bone loss and strength. Loss of estrogens or androgens accelerates the effects of aging on bone by decreasing defense against oxidative stress.
Figures

References
-
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Osteoporosis Int. 1998;8:468–489. - PubMed
-
- Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. Calcif. Tissue Int. 1985;37:594–597. - PubMed
-
- Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R. J. Biomech. 1998;31:337–345. - PubMed
-
- Heaney RP. Bone. 2003;33:457–465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

