Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors
- PMID: 17624413
- PMCID: PMC2100381
- DOI: 10.1016/j.yfrne.2007.05.003
Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors
Abstract
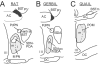
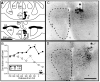
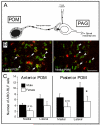
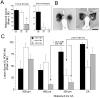
Several studies have suggested dissociations between neural circuits underlying the expression of appetitive (e.g., courtship behavior) and consummatory components (i.e., copulatory behavior) of vertebrate male sexual behavior. The medial preoptic area (mPOA) clearly controls the expression of male copulation but, according to a number of experiments, is not necessarily implicated in the expression of appetitive sexual behavior. In rats for example, lesions to the mPOA eliminate male-typical copulatory behavior but have more subtle or no obvious effects on measures of sexual motivation. Rats with such lesions still pursue and attempt to mount females. They also acquire and perform learned instrumental responses to gain access to females. However, recent lesions studies and measures of the expression of the immediate early gene c-fos demonstrate that, in quail, sub-regions of the mPOA, in particular of its sexually dimorphic component the medial preoptic nucleus, can be specifically linked with either the expression of appetitive or consummatory sexual behavior. In particular more rostral regions can be linked to appetitive components while more caudal regions are involved in consummatory behavior. This functional sub-region variation is associated with neurochemical and hodological specializations (i.e., differences in chemical phenotype of the cells or in their connectivity), especially those related to the actions of androgens in relation to the activation of male sexual behavior, that are also present in rodents and other species. It could thus reflect general principles about POA organization and function in the vertebrate brain.
Figures

Similar articles
-
Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior.Brain Res Bull. 2000 Jul 1;52(4):249-62. doi: 10.1016/s0361-9230(00)00233-1. Brain Res Bull. 2000. PMID: 10856822
-
The role of the medial preoptic area in appetitive and consummatory reproductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters.Neuroscience. 2010 Nov 10;170(4):1120-32. doi: 10.1016/j.neuroscience.2010.08.029. Epub 2010 Aug 21. Neuroscience. 2010. PMID: 20732389 Free PMC article.
-
Performance of appetitive or consummatory components of male sexual behavior is mediated by different brain areas: a 2-deoxyglucose autoradiographic study.Neuroscience. 1999;94(4):1261-77. doi: 10.1016/s0306-4522(99)00318-8. Neuroscience. 1999. PMID: 10625066
-
Hormonal regulation of brain circuits mediating male sexual behavior in birds.Physiol Behav. 2004 Nov 15;83(2):329-46. doi: 10.1016/j.physbeh.2004.08.020. Physiol Behav. 2004. PMID: 15488549 Review.
-
Medial preoptic area/anterior hypothalamus and sexual motivation.Scand J Psychol. 2003 Jul;44(3):203-12. doi: 10.1111/1467-9450.00337. Scand J Psychol. 2003. PMID: 12914583 Review.
Cited by
-
Wired for behaviors: from development to function of innate limbic system circuitry.Front Mol Neurosci. 2012 Apr 26;5:55. doi: 10.3389/fnmol.2012.00055. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22557946 Free PMC article.
-
Role of aromatase in distinct brain nuclei of the social behaviour network in the expression of sexual behaviour in male Japanese quail.J Neuroendocrinol. 2022 Jun;34(6):e13127. doi: 10.1111/jne.13127. Epub 2022 Apr 8. J Neuroendocrinol. 2022. PMID: 35394094 Free PMC article.
-
Differential disruption of conditioned ejaculatory preference in the male rat based on different sensory modalities by micro-infusions of naloxone to the medial preoptic area or ventral tegmental area.Psychopharmacology (Berl). 2019 Dec;236(12):3613-3623. doi: 10.1007/s00213-019-05334-9. Epub 2019 Jul 29. Psychopharmacology (Berl). 2019. PMID: 31359118
-
Dopamine, Erectile Function and Male Sexual Behavior from the Past to the Present: A Review.Brain Sci. 2022 Jun 24;12(7):826. doi: 10.3390/brainsci12070826. Brain Sci. 2022. PMID: 35884633 Free PMC article. Review.
-
Status-appropriate singing behavior, testosterone and androgen receptor immunolabeling in male European starlings (Sturnus vulgaris).Horm Behav. 2014 Apr;65(4):329-39. doi: 10.1016/j.yhbeh.2014.02.010. Epub 2014 Mar 1. Horm Behav. 2014. PMID: 24594286 Free PMC article.
References
-
- Absil P, Riters LV, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male Japanese quail (Coturnix japonica) Horm. Behav. 2001 - PubMed
-
- Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav. Evol. 2002;60:13–35. - PubMed
-
- Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J. Comp. Physiol. Psychol. 1975;89:61–71. - PubMed
-
- Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pniewski EE. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol. Behav. 1980;24:441–446. - PubMed
-
- Adkins-Regan E. Hormones and animal social behavior. Princeton University Press; Princeton, NJ: 2005. p. 411.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

