Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice
- PMID: 17624463
- PMCID: PMC2134841
- DOI: 10.1016/j.virusres.2007.05.022
Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice
Abstract
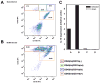
Murine cytomegalovirus (MCMV) brain infection induces a transient increase in chemokine production, which precedes the infiltration of CD3(+) lymphocytes. In this study, we hypothesized that an absence of anti-inflammatory cytokines would result in sustained proinflammatory neuroimmune responses. Direct intracerebroventricular injection of MCMV into IL-10 knockout (KO) mice produced an unexpected result: while wild-type animals controlled MCMV, the infection was lethal in IL-10 KO animals. Identical infection of IL-4 KO animals did not produce lethal disease. To further characterize the role of IL-10, infected brain tissue from both wild-type and IL-10 KO animals was assessed for cytokine and chemokine levels, as well as viral gene expression. These data show vastly elevated levels of interferon (IFN)-gamma, and the IFN-gamma-inducible chemokines CXCL9 and CXCL10, as well as IL-6 in brain homogenates obtained from IL-10 KO animals. However, MCMV viral load, glycoprotein B mRNA levels, and titers of infectious virus were similar in both IL-10 KO and wild-type animals. Separation of cells isolated from murine brain tissue into distinct populations using FACS, along with subsequent quantitative RT real-time PCR, showed that brain-infiltrating CD45(hi)/CD11b(-) and CD45(hi)/CD11b(int) were the cellular source of IL-10 in the brain. Taken together, these data demonstrate that MCMV brain infection of IL-10-deficient mice causes lethal disease, which occurs in the presence of a dysregulated IFN-gamma-mediated neuroimmune response.
Figures



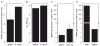
References
-
- Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11(10):1625–34. - PubMed
-
- Chao CC, Hu S, Sheng WS, Peterson PK. Tumor necrosis factor-alpha production by human fetal microglial cells: regulation by other cytokines. Dev Neurosci. 1995;17(2):97–105. - PubMed
-
- Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J Neurovirol. 2004;10(3):152–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

