Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons
- PMID: 17624620
- PMCID: PMC2725433
- DOI: 10.1007/s10735-007-9112-7
Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons
Abstract
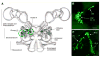
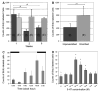
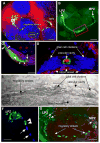
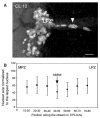
Adult neurogenesis is a characteristic feature of the olfactory pathways of decapod crustaceans. In crayfish and clawed lobsters, adult-born neurons are the progeny of precursor cells with glial characteristics located in a neurogenic niche on the ventral surface of the brain. The daughters of these precursor cells migrate during S and G(2 )stages of the cell cycle along glial fibers to lateral (cluster 10) and medial (cluster 9) proliferation zones. Here, they divide (M phase) producing offspring that differentiate into olfactory interneurons. The complete lineage of cells producing neurons in these animals, therefore, is arranged along the migratory stream according to cell cycle stage. We have exploited this model to examine the influence of environmental and endogenous factors on adult neurogenesis. We find that increased levels of serotonin upregulate neuronal production, as does maintaining animals in an enriched (versus deprived) environment or augmenting their diet with omega-3 fatty acids; increased levels of nitric oxide, on the other hand, decrease the rate of neurogenesis. The features of the neurogenic niche and migratory streams, and the fact that these continue to function in vitro, provide opportunities unavailable in other organisms to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains.
Figures
Similar articles
-
First-generation neuronal precursors in the crayfish brain are not self-renewing.Int J Dev Neurosci. 2013 Nov;31(7):657-66. doi: 10.1016/j.ijdevneu.2012.11.010. Epub 2012 Dec 5. Int J Dev Neurosci. 2013. PMID: 23219763 Free PMC article. Review.
-
Adult neurogenesis in the crayfish brain: proliferation, migration, and possible origin of precursor cells.Dev Neurobiol. 2009 Jun;69(7):415-36. doi: 10.1002/dneu.20717. Dev Neurobiol. 2009. PMID: 19294644 Free PMC article.
-
Adult neurogenesis: a common strategy across diverse species.J Comp Neurol. 2007 Jan 20;500(3):574-84. doi: 10.1002/cne.21187. J Comp Neurol. 2007. PMID: 17120293 Free PMC article.
-
Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb.J Neurosci. 2004 Jan 7;24(1):85-95. doi: 10.1523/JNEUROSCI.1574-03.2004. J Neurosci. 2004. PMID: 14715941 Free PMC article.
-
The olfactory pathway of decapod crustaceans--an invertebrate model for life-long neurogenesis.Chem Senses. 2007 May;32(4):365-84. doi: 10.1093/chemse/bjm008. Epub 2007 Apr 2. Chem Senses. 2007. PMID: 17404151 Review.
Cited by
-
Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection?Eur J Neurosci. 2011 Sep;34(6):870-83. doi: 10.1111/j.1460-9568.2011.07802.x. Eur J Neurosci. 2011. PMID: 21929622 Free PMC article. Review.
-
Adult neurogenesis: examples from the decapod crustaceans and comparisons with mammals.Arthropod Struct Dev. 2011 May;40(3):258-75. doi: 10.1016/j.asd.2011.03.001. Epub 2011 Mar 9. Arthropod Struct Dev. 2011. PMID: 21396485 Free PMC article. Review.
-
First-generation neuronal precursors in the crayfish brain are not self-renewing.Int J Dev Neurosci. 2013 Nov;31(7):657-66. doi: 10.1016/j.ijdevneu.2012.11.010. Epub 2012 Dec 5. Int J Dev Neurosci. 2013. PMID: 23219763 Free PMC article. Review.
-
Nitric oxide in the crustacean brain: regulation of neurogenesis and morphogenesis in the developing olfactory pathway.Dev Dyn. 2007 Nov;236(11):3047-60. doi: 10.1002/dvdy.21340. Dev Dyn. 2007. PMID: 17948307 Free PMC article.
-
Growth patterns in Onychophora (velvet worms): lack of a localised posterior proliferation zone.BMC Evol Biol. 2010 Nov 4;10:339. doi: 10.1186/1471-2148-10-339. BMC Evol Biol. 2010. PMID: 21050428 Free PMC article.
References
-
- Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. - PubMed
-
- Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential Cell Biology. 18. Vol. 17. Garland Publishing, Inc; NY: 1998.
-
- Allodi S, Bressan CM, Carvalho SL, Cavalcante LA. Regionally specific distribution of the binding of anti-glutamate synthetase and anti-S100 antibodies and of Datura stratonium lectin in glial domains of the optic lobe of the giant prawn. Glia. 2006;53:612–620. - PubMed
-
- Arbas EA, Humphreys CJ, Ache BW. Morphology and physiological properties of interneurons in the olfactory midbrain of the crayfish. J Comp Physiol A. 1988;164:231–241. - PubMed
-
- Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

