Inhibition of HIV-1 replication by P-TEFb inhibitors DRB, seliciclib and flavopiridol correlates with release of free P-TEFb from the large, inactive form of the complex
- PMID: 17625008
- PMCID: PMC1948018
- DOI: 10.1186/1742-4690-4-47
Inhibition of HIV-1 replication by P-TEFb inhibitors DRB, seliciclib and flavopiridol correlates with release of free P-TEFb from the large, inactive form of the complex
Abstract
Background: The positive transcription elongation factor, P-TEFb, comprised of cyclin dependent kinase 9 (Cdk9) and cyclin T1, T2 or K regulates the productive elongation phase of RNA polymerase II (Pol II) dependent transcription of cellular and integrated viral genes. P-TEFb containing cyclin T1 is recruited to the HIV long terminal repeat (LTR) by binding to HIV Tat which in turn binds to the nascent HIV transcript. Within the cell, P-TEFb exists as a kinase-active, free form and a larger, kinase-inactive form that is believed to serve as a reservoir for the smaller form.
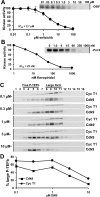
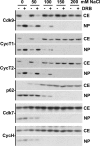
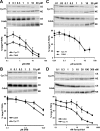
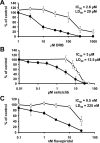
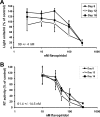
Results: We developed a method to rapidly quantitate the relative amounts of the two forms based on differential nuclear extraction. Using this technique, we found that titration of the P-TEFb inhibitors flavopiridol, DRB and seliciclib onto HeLa cells that support HIV replication led to a dose dependent loss of the large form of P-TEFb. Importantly, the reduction in the large form correlated with a reduction in HIV-1 replication such that when 50% of the large form was gone, HIV-1 replication was reduced by 50%. Some of the compounds were able to effectively block HIV replication without having a significant impact on cell viability. The most effective P-TEFb inhibitor flavopiridol was evaluated against HIV-1 in the physiologically relevant cell types, peripheral blood lymphocytes (PBLs) and monocyte derived macrophages (MDMs). Flavopiridol was found to have a smaller therapeutic index (LD50/IC50) in long term HIV-1 infectivity studies in primary cells due to greater cytotoxicity and reduced efficacy at blocking HIV-1 replication.
Conclusion: Initial short term studies with P-TEFb inhibitors demonstrated a dose dependent loss of the large form of P-TEFb within the cell and a concomitant reduction in HIV-1 infectivity without significant cytotoxicity. These findings suggested that inhibitors of P-TEFb may serve as effective anti-HIV-1 therapies. However, longer term HIV-1 replication studies indicated that these inhibitors were more cytotoxic and less efficacious against HIV-1 in the primary cell cultures.
Figures
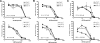
Similar articles
-
Identification of flavopiridol analogues that selectively inhibit positive transcription elongation factor (P-TEFb) and block HIV-1 replication.Chembiochem. 2009 Aug 17;10(12):2072-80. doi: 10.1002/cbic.200900303. Chembiochem. 2009. PMID: 19603446 Free PMC article.
-
Flavopiridol inhibits P-TEFb and blocks HIV-1 replication.J Biol Chem. 2000 Sep 15;275(37):28345-8. doi: 10.1074/jbc.C000446200. J Biol Chem. 2000. PMID: 10906320
-
Role of the HIV-1 positive elongation factor P-TEFb and inhibitors thereof.Mini Rev Med Chem. 2009 Mar;9(3):379-85. doi: 10.2174/1389557510909030379. Mini Rev Med Chem. 2009. PMID: 19275730 Review.
-
Indirubin-3'-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication.AIDS. 2005 Dec 2;19(18):2087-95. doi: 10.1097/01.aids.0000194805.74293.11. AIDS. 2005. PMID: 16284457
-
CDK9 a potential target for drug development.Med Chem. 2008 May;4(3):210-8. doi: 10.2174/157340608784325205. Med Chem. 2008. PMID: 18473913 Review.
Cited by
-
Identification of new drug treatments to combat COVID19: A signature-based approach using iLINCS.Res Sq [Preprint]. 2020 Apr 30:rs.3.rs-25643. doi: 10.21203/rs.3.rs-25643/v1. Res Sq. 2020. PMID: 32702077 Free PMC article. Preprint.
-
The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells.PLoS One. 2013;8(1):e55684. doi: 10.1371/journal.pone.0055684. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383264 Free PMC article.
-
The Role of RNA Polymerase II Elongation Control in HIV-1 Gene Expression, Replication, and Latency.Genet Res Int. 2011;2011:726901. doi: 10.4061/2011/726901. Epub 2011 Oct 13. Genet Res Int. 2011. PMID: 22567366 Free PMC article.
-
New insights into the control of HIV-1 transcription: when Tat meets the 7SK snRNP and super elongation complex (SEC).J Neuroimmune Pharmacol. 2011 Jun;6(2):260-8. doi: 10.1007/s11481-011-9267-6. Epub 2011 Mar 1. J Neuroimmune Pharmacol. 2011. PMID: 21360054 Free PMC article. Review.
-
Transcriptional Elongation of HSV Immediate Early Genes by the Super Elongation Complex Drives Lytic Infection and Reactivation from Latency.Cell Host Microbe. 2017 Apr 12;21(4):507-517.e5. doi: 10.1016/j.chom.2017.03.007. Cell Host Microbe. 2017. PMID: 28407486 Free PMC article.
References
-
- Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol Cell Biol. 2004;24:787–795. doi: 10.1128/MCB.24.2.787-795.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

