Familial breast/ovarian cancer and BRCA1/2 genetic screening: the role of immunohistochemistry as an additional method in the selection of patients
- PMID: 17625228
- PMCID: PMC3957528
- DOI: 10.1369/jhc.7A7209.2007
Familial breast/ovarian cancer and BRCA1/2 genetic screening: the role of immunohistochemistry as an additional method in the selection of patients
Abstract
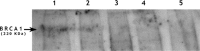
Only 20-25% of families screened for BRCA1/2 mutations are found positive. Because only a positive result is informative, we studied the role of BRCA1/2 immunohistochemistry as an additional method for patient selection. From 53 high-risk-affected probands, 18 (34%) had available paraffin blocks of their tumors and were selected for this study. Mutation screening was done by conformation-sensitive gel electrophoresis and multiplex ligation-dependent probe amplification. For immunohistochemistry, 21 neoplastic specimens (15 breast carcinomas, 5 ovary neoplasms, and 1 rectal adenocarcinoma) were analyzed with BRCA1 (monoclonal antibody, Ab-1, oncogene) and BRCA2 (polyclonal antibody, Ab-2, oncogene) antibodies. Absence of the BRCA1 protein was confirmed in negative tumors by Western blotting. Seven patients were positive for BRCA1/2 mutations: 5 for BRCA1 and 2 for BRCA2. Four out of five positive patients had tumors negative for BRCA1 immunostaining, and the remaining 13 BRCA1-negative patients had positive BRCA1 immunostaining in all tumor samples. Sensitivity to predict for BRCA1 mutation carriers was 80%, and specificity was 100%, with a positive predictive value of 100% and a negative predictive value of 93%. This correlation was statistically significant (p=0.001). No correlation was observed for BRCA2. If larger studies confirm these results, high-risk patients with BRCA1-negative tumors should be screened first for this gene.
Figures

References
-
- Al-Mulla F, Abdulrahman M, Varadharaj G, Akhter N, Anim JT. (2005) BRCA1 gene expression in breast cancer: a correlative study between real-time RT-PCR and immunohistochemistry. J Histochem Cytochem 53: 621–629 - PubMed
-
- American Society of Clinical Oncology (2003) American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 21: 2397–2406 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Breast Cancer Linkage Consortium (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91: 1310–1316 - PubMed
-
- Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. (2002) Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94: 1365–1372 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

