Gene therapy trials for the treatment of high-grade gliomas
- PMID: 17625614
- PMCID: PMC1913943
Gene therapy trials for the treatment of high-grade gliomas
Abstract
High-grade gliomas remain relatively resistant to current therapy. Local recurrence is a common feature and the majority of patients progress despite conventional therapy. One modality-gene therapy-has shown a lot of promise in early preclinical and clinical studies aimed at advancing the treatment of this disease. In this review, we provide a comprehensive overview of clinical trials involving gene therapy in the field of neuro-oncology. The use of different delivery vehicles, including liposomes, cells, and viruses, as well genes, especially cytokines and suicide genes, are explored in detail. The unique features and advantages/disadvantages of the different vectors employed are compared based on results of human studies. We discuss both the limitations and successes encountered in these clinical trials, with an emphasis on the lessons learned and potential ways of improving current gene therapy protocols.
Figures

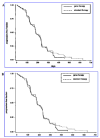

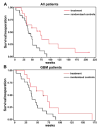
References
-
- Andrews DW, Resnicoff M, Flanders AE, Kenyon L, Curtis M, Merli G, Baserga R, Iliakis G, Aiken RD. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19:2189–2200. - PubMed
-
- Annegers JF, Schoenberg BS, Okazaki H, Kurland LT. Epidemiologic study of primary intracranial neoplasms. Arch Neurol. 1981;38:217–219. - PubMed
-
- Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the Coxsackie virus and adenovirus receptor. J Neurosurg. 2000;92:1002–1008. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
