A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems
- PMID: 17626000
- PMCID: PMC2093964
- DOI: 10.1098/rsif.2007.1089
A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems
Abstract
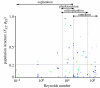
The fluid mechanics of marine and terrestrial systems are surprisingly similar at many spatial and temporal scales. Not surprisingly, the dispersal of organisms that float, swim or fly is influenced by the fluid environments of air and seawater. Nonetheless, it has been argued repeatedly that the geography of evolution differs fundamentally between marine and terrestrial taxa. Might this view emanate from qualitative contrasts between the pelagic ocean and terrestrial land conflated by anthropocentric perception of within- and between-realm variation? We draw on recent advances in biogeography to identify two pairs of biophysically similar marine and terrestrial settings--(i) aerial and marine microplankton and (ii) true islands and brackish seawater lakes--which have similar geographies of evolution. Commonalities at these scales, the largest and smallest biogeographic scales, delimit the geographical extents that can possibly characterize evolution in the remaining majority of species. The geographies of evolution therefore differ statistically, not fundamentally, between marine and terrestrial systems. Comparing the geography of evolution in diverse non-microplanktonic and non-island species from a biophysical perspective is an essential next step for quantifying precisely how marine and terrestrial systems differ and is an important yet under-explored avenue of macroecology.
Figures


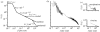
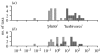
Similar articles
-
Controls on diatom biogeography in the ocean.Science. 2009 Sep 18;325(5947):1539-41. doi: 10.1126/science.1174159. Science. 2009. PMID: 19762642
-
Genomic evidence for global ocean plankton biogeography shaped by large-scale current systems.Elife. 2022 Aug 3;11:e78129. doi: 10.7554/eLife.78129. Elife. 2022. PMID: 35920817 Free PMC article.
-
Global Trends in Marine Plankton Diversity across Kingdoms of Life.Cell. 2019 Nov 14;179(5):1084-1097.e21. doi: 10.1016/j.cell.2019.10.008. Cell. 2019. PMID: 31730851 Free PMC article.
-
Long-term oceanographic and ecological research in the Western English Channel.Adv Mar Biol. 2005;47:1-105. doi: 10.1016/S0065-2881(04)47001-1. Adv Mar Biol. 2005. PMID: 15596166 Review.
-
Impacts of climate change on marine organisms and ecosystems.Curr Biol. 2009 Jul 28;19(14):R602-14. doi: 10.1016/j.cub.2009.05.046. Curr Biol. 2009. PMID: 19640499 Review.
Cited by
-
Why are there so few fish in the sea?Proc Biol Sci. 2012 Jun 22;279(1737):2323-9. doi: 10.1098/rspb.2012.0075. Epub 2012 Feb 8. Proc Biol Sci. 2012. PMID: 22319126 Free PMC article.
-
Population structure and phylogeography reveal pathways of colonization by a migratory marine reptile (Chelonia mydas) in the central and eastern Pacific.Ecol Evol. 2014 Nov;4(22):4317-31. doi: 10.1002/ece3.1269. Epub 2014 Oct 25. Ecol Evol. 2014. PMID: 25540693 Free PMC article.
-
Phylogenetics and Population Genetics of the Petrolisthes lamarckii-P. haswelli Complex in China: Old Lineage and New Species.Int J Mol Sci. 2023 Oct 31;24(21):15843. doi: 10.3390/ijms242115843. Int J Mol Sci. 2023. PMID: 37958829 Free PMC article.
-
A profile of an endosymbiont-enriched fraction of the coral Stylophora pistillata reveals proteins relevant to microbial-host interactions.Mol Cell Proteomics. 2012 Jun;11(6):M111.015487. doi: 10.1074/mcp.M111.015487. Epub 2012 Feb 20. Mol Cell Proteomics. 2012. PMID: 22351649 Free PMC article.
-
Scaling the extinction vortex: Body size as a predictor of population dynamics close to extinction events.Ecol Evol. 2021 May 2;11(11):7069-7079. doi: 10.1002/ece3.7555. eCollection 2021 Jun. Ecol Evol. 2021. PMID: 34141276 Free PMC article.
References
-
- Addicott W.O. Late Pleistocene marine paleoecology and zoogeography in central California. US Geol. Surv. Prof. Pap. 1966;523C:C1–C21.
-
- Aleyev Y.G. Jung; The Hague, The Netherlands: 1977. Nekton.
-
- Angel M.V. Biodiversity of the pelagic ocean. Conserv. Biol. 1993;7:760–772. doi: 10.1046/j.1523-1739.1993.740760.x. - DOI
-
- Baldwin A.J, Moss J.A, Pakulski J.D, Catala P, Joux F, Jeffrey W.H. Microbial diversity in a Pacific Ocean transect from the Arctic to Antarctic circles. Aquat. Microb. Ecol. 2005;41:91–102.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

