Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response
- PMID: 17626075
- PMCID: PMC2045380
- DOI: 10.1128/JVI.00792-07
Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response
Abstract
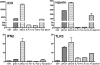
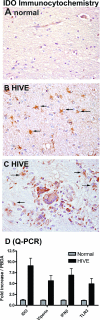
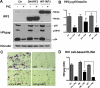
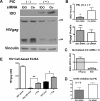
Indoleamine 2,3-dioxygenase (IDO) is the first and rate-limiting enzyme in the kynurenine pathway of tryptophan catabolism and has been implicated in neurotoxicity and suppression of the antiviral T-cell response in HIV encephalitis (HIVE). Here we show that the Toll-like receptor 3 (TLR3) ligand poly(I:C) (PIC) induces the expression of IDO in human astrocytes. PIC was less potent than gamma interferon (IFN-gamma) but more potent than IFN-beta in inducing IDO. PIC induction of IDO was mediated in part by IFN-beta but not IFN-gamma, and both NF-kappaB and interferon regulatory factor 3 (IRF3) were required. PIC also upregulated TLR3, thereby augmenting the primary (IFN-beta) and secondary (IDO and viperin) response genes upon subsequent stimulation with PIC. In HIVE, the transcripts for TLR3, IFN-beta, IDO, and viperin were increased and IDO immunoreactivity was detected in reactive astrocytes as well as macrophages and microglia. PIC caused suppression of intracellular replication of human immunodeficiency virus pseudotyped with vesicular stomatitis virus G protein and human cytomegalovirus in a manner dependent on IRF3 and IDO. The involvement of IDO was demonstrated by partial but significant reversal of the PIC-mediated antiviral effect by IDO RNA interference and/or tryptophan supplementation. Importantly, the cytokine interleukin-1 abolished IFN-gamma-induced IDO enzyme activity in a nitric oxide-dependent manner without suppressing protein expression. Our results demonstrate that IDO is an innate antiviral protein induced by double-stranded RNA and suggest a therapeutic utility for PIC in human viral infections. They also show that IDO activity can be dissociated from protein expression, indicating that the local central nervous system cytokine and nitric oxide environment determines IDO function.
Figures





Similar articles
-
TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5.J Immunol. 2006 Oct 1;177(7):4735-41. doi: 10.4049/jimmunol.177.7.4735. J Immunol. 2006. PMID: 16982913
-
Flavivirus infection induces indoleamine 2,3-dioxygenase in human monocyte-derived macrophages via tumor necrosis factor and NF-κB.J Leukoc Biol. 2012 Apr;91(4):657-66. doi: 10.1189/jlb.1011532. Epub 2012 Feb 1. J Leukoc Biol. 2012. PMID: 22301793
-
Toll-like receptor-3 ligation-induced indoleamine 2, 3-dioxygenase expression in human trophoblasts.Endocrinology. 2011 Dec;152(12):4984-92. doi: 10.1210/en.2011-0278. Epub 2011 Sep 27. Endocrinology. 2011. PMID: 21952237
-
Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging.Ann N Y Acad Sci. 2007 Dec;1122:35-49. doi: 10.1196/annals.1403.003. Ann N Y Acad Sci. 2007. PMID: 18077563 Review.
-
IDO expression in the brain: a double-edged sword.J Mol Med (Berl). 2007 Dec;85(12):1351-9. doi: 10.1007/s00109-007-0229-7. Epub 2007 Jun 27. J Mol Med (Berl). 2007. PMID: 17594069 Review.
Cited by
-
Does the kynurenine pathway play a pathogenic role in autism spectrum disorder?Brain Behav Immun Health. 2024 Aug 6;40:100839. doi: 10.1016/j.bbih.2024.100839. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39263315 Free PMC article.
-
Blockage of indoleamine 2,3-dioxygenase regulates Japanese encephalitis via enhancement of type I/II IFN innate and adaptive T-cell responses.J Neuroinflammation. 2016 Apr 18;13(1):79. doi: 10.1186/s12974-016-0551-5. J Neuroinflammation. 2016. PMID: 27090635 Free PMC article.
-
Physiology of Astroglia.Physiol Rev. 2018 Jan 1;98(1):239-389. doi: 10.1152/physrev.00042.2016. Physiol Rev. 2018. PMID: 29351512 Free PMC article. Review.
-
Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway.Virology. 2013 Nov;446(1-2):199-206. doi: 10.1016/j.virol.2013.08.005. Epub 2013 Aug 30. Virology. 2013. PMID: 24074582 Free PMC article.
-
Evidence of the innate antiviral and neuroprotective properties of progranulin.PLoS One. 2014 May 30;9(5):e98184. doi: 10.1371/journal.pone.0098184. eCollection 2014. PLoS One. 2014. PMID: 24878635 Free PMC article.
References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Anderson, C. E., G. S. Tomlinson, B. Pauly, F. W. Brannan, A. Chiswick, R. Brack-Werner, P. Simmonds, and J. E. Bell. 2003. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol. Appl. Neurobiol. 29:378-388. - PubMed
-
- Barber, S. A., D. S. Herbst, B. T. Bullock, L. Gama, and J. E. Clements. 2004. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol. 10(Suppl. 1):15-20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

