Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo
- PMID: 17626092
- PMCID: PMC2045394
- DOI: 10.1128/JVI.00530-07
Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo
Abstract
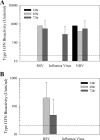
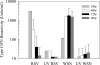
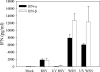
Type I interferon (IFN) induction is an immediate response to virus infection, and very high levels of these cytokines are produced when the Toll-like receptors (TLRs) expressed at high levels by plasmacytoid dendritic cells (pDCs) are triggered by viral nucleic acids. Unlike many RNA viruses, respiratory syncytial virus (RSV) does not appear to activate pDCs through their TLRs and it is not clear how this difference affects IFN-alpha/beta induction in vivo. In this study, we investigated type I IFN production triggered by RSV or influenza A virus infection of BALB/c mice and found that while both viruses induced IFN-alpha/beta production by pDCs in vitro, only influenza virus infection could stimulate type I IFN synthesis by pDCs in vivo. In situ hybridization studies demonstrated that the infected respiratory epithelium was a major source of IFN-alpha/beta in response to either infection, but in pDC-depleted animals only type I IFN induction by influenza virus was impaired.
Figures







References
-
- Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Brière, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. - PubMed
-
- Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

