CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis
- PMID: 17626103
- PMCID: PMC2045405
- DOI: 10.1128/JVI.00941-07
CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis
Abstract
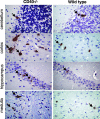
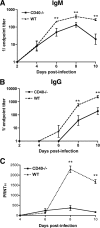
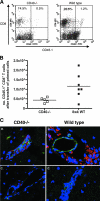
Recent studies have established a protective role for T cells during primary West Nile virus (WNV) infection. Binding of CD40 by CD40 ligand (CD40L) on activated CD4+ T cells provides an important costimulatory signal for immunoglobulin class switching, antibody affinity maturation, and priming of CD8+ T-cell responses. We examined here the function of CD40-dependent interactions in limiting primary WNV infection. Compared to congenic wild-type mice, CD40(-/-) mice uniformly succumbed to WNV infection. Although CD40(-/-) mice produced low levels of WNV-specific immunoglobulin M (IgM) and IgG, viral clearance from the spleen and serum was not altered, and CD8+ T-cell priming in peripheral lymphoid tissues was normal. Unexpectedly, CD8+ T-cell trafficking to the central nervous system (CNS) was markedly impaired in CD40(-/-) mice, and this correlated with elevated WNV titers in the CNS and death. In the brains of CD40(-/-) mice, T cells were retained in the perivascular space and did not migrate into the parenchyma, the predominant site of WNV infection. In contrast, in wild-type mice, T cells trafficked to the site of infection in neurons. Beside its role in maturation of antibody responses, our experiments suggest a novel function of CD40-CD40L interactions: to facilitate T-cell migration across the blood-brain barrier to control WNV infection.
Figures




Similar articles
-
CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system.J Virol. 2006 Dec;80(24):12060-9. doi: 10.1128/JVI.01650-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035323 Free PMC article.
-
CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons.J Virol. 2012 Sep;86(17):8937-48. doi: 10.1128/JVI.00673-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740407 Free PMC article.
-
IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis.J Immunol. 2014 Oct 15;193(8):4095-106. doi: 10.4049/jimmunol.1401192. Epub 2014 Sep 8. J Immunol. 2014. PMID: 25200953 Free PMC article.
-
Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor.Viral Immunol. 2019 Jan/Feb;32(1):38-47. doi: 10.1089/vim.2018.0075. Epub 2018 Sep 15. Viral Immunol. 2019. PMID: 30222521 Review.
-
CD8 and CD4 T cells in west nile virus immunity and pathogenesis.Viruses. 2013 Oct 22;5(10):2573-84. doi: 10.3390/v5102573. Viruses. 2013. PMID: 24153060 Free PMC article. Review.
Cited by
-
MHC class Ib-restricted CD8 T cells differ in dependence on CD4 T cell help and CD28 costimulation over the course of mouse polyomavirus infection.J Immunol. 2012 Apr 1;188(7):3071-9. doi: 10.4049/jimmunol.1103554. Epub 2012 Mar 5. J Immunol. 2012. PMID: 22393155 Free PMC article.
-
Differential virulence and pathogenesis of West Nile viruses.Viruses. 2013 Nov 22;5(11):2856-80. doi: 10.3390/v5112856. Viruses. 2013. PMID: 24284878 Free PMC article. Review.
-
Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors.J Neurosci. 2012 Oct 17;32(42):14489-510. doi: 10.1523/JNEUROSCI.1256-12.2012. J Neurosci. 2012. PMID: 23077035 Free PMC article.
-
CD22 is required for protection against West Nile virus Infection.J Virol. 2013 Mar;87(6):3361-75. doi: 10.1128/JVI.02368-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302871 Free PMC article.
-
CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease?J Neuroimmune Pharmacol. 2009 Dec;4(4):462-75. doi: 10.1007/s11481-009-9166-2. Epub 2009 Aug 11. J Neuroimmune Pharmacol. 2009. PMID: 19669892 Free PMC article. Review.
References
-
- Abromson-Leeman, S., E. Maverakis, R. Bronson, and M. E. Dorf. 2001. CD40-mediated activation of T cells accelerates, but is not required for, encephalitogenic potential of myelin basic protein-recognizing T cells in a model of progressive experimental autoimmune encephalomyelitis. Eur. J. Immunol. 31:527-538. - PubMed
-
- Bachmann, M. F., L. Hunziker, R. M. Zinkernagel, T. Storni, and M. Kopf. 2004. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur. J. Immunol. 34:317-326. - PubMed
-
- Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

