Type I interferon inhibition and dendritic cell activation during gammaherpesvirus respiratory infection
- PMID: 17626106
- PMCID: PMC2045419
- DOI: 10.1128/JVI.00360-07
Type I interferon inhibition and dendritic cell activation during gammaherpesvirus respiratory infection
Abstract
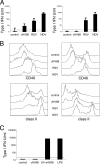
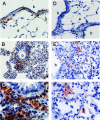
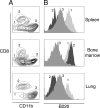
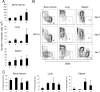
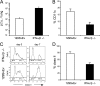
The respiratory tract is a major mucosal site for microorganism entry into the body, and type I interferon (IFN) and dendritic cells constitute a first line of defense against viral infections. We have analyzed the interaction between a model DNA virus, plasmacytoid dendritic cells, and type I IFN during lung infection of mice. Our data show that murine gammaherpesvirus 68 (gammaHV68) inhibits type I IFN secretion by dendritic cells and that plasmacytoid dendritic cells are necessary for conventional dendritic cell maturation in response to gammaHV68. Following gammaHV68 intranasal inoculation, the local and systemic IFN-alpha/beta response is below detectable levels, and plasmacytoid dendritic cells are activated and recruited into the lung with a tissue distribution that differs from that of conventional dendritic cells. Our results suggest that plasmacytoid dendritic cells and type I IFN have important but independent roles during the early response to a respiratory gammaHV68 infection. gammaHV68 infection inhibits type I IFN production by dendritic cells and is a poor inducer of IFN-alpha/beta in vivo, which may serve as an immune evasion strategy.
Figures

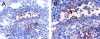


Similar articles
-
Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner.J Virol. 2007 Jun;81(11):6134-40. doi: 10.1128/JVI.00108-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360749 Free PMC article. Review.
-
Type I Interferon Signaling to Dendritic Cells Limits Murid Herpesvirus 4 Spread from the Olfactory Epithelium.J Virol. 2017 Nov 14;91(23):e00951-17. doi: 10.1128/JVI.00951-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904198 Free PMC article.
-
A Rhesus Rhadinovirus Viral Interferon (IFN) Regulatory Factor Is Virion Associated and Inhibits the Early IFN Antiviral Response.J Virol. 2015 Aug;89(15):7707-21. doi: 10.1128/JVI.01175-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972548 Free PMC article.
-
Independent of plasmacytoid dendritic cell (pDC) infection, pDC triggered by virus-infected cells mount enhanced type I IFN responses of different composition as opposed to pDC stimulated with free virus.J Immunol. 2014 Sep 1;193(5):2496-503. doi: 10.4049/jimmunol.1400215. Epub 2014 Jul 28. J Immunol. 2014. PMID: 25070849
-
Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation.Leuk Lymphoma. 2004 Feb;45(2):257-64. doi: 10.1080/1042819031000149368. Leuk Lymphoma. 2004. PMID: 15101709 Review.
Cited by
-
Suppression of TLR9 immunostimulatory motifs in the genome of a gammaherpesvirus.J Immunol. 2011 Jul 15;187(2):887-96. doi: 10.4049/jimmunol.1003737. Epub 2011 Jun 10. J Immunol. 2011. PMID: 21666062 Free PMC article.
-
Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity.Proc Natl Acad Sci U S A. 2012 May 1;109(18):E1092-100. doi: 10.1073/pnas.1203019109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474381 Free PMC article.
-
Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection.J Virol. 2012 May;86(10):5422-36. doi: 10.1128/JVI.06757-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398282 Free PMC article.
-
A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality.PLoS Pathog. 2011 Nov;7(11):e1002371. doi: 10.1371/journal.ppat.1002371. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114555 Free PMC article.
-
North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells.J Virol. 2011 Mar;85(6):2703-13. doi: 10.1128/JVI.01616-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191013 Free PMC article.
References
-
- Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. - PubMed
-
- Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. - PubMed
-
- Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711-6719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

