Testing of four Leishmania vaccine candidates in a mouse model of infection with Leishmania (Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World
- PMID: 17626159
- PMCID: PMC2043302
- DOI: 10.1128/CVI.00060-07
Testing of four Leishmania vaccine candidates in a mouse model of infection with Leishmania (Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World
Abstract
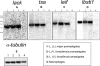
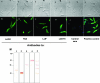
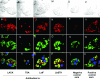
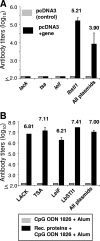
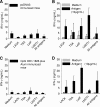
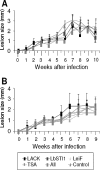
We evaluated whether four recombinant antigens previously used for vaccination against experimental infection with Leishmania (Leishmania) major could also induce protective immunity against a challenge with Leishmania (Viannia) braziliensis, the species responsible for 90% of the 28,712 annual cases of cutaneous and mucocutaneous leishmaniasis recorded in Brazil during the year of 2004. Initially, we isolated the homolog genes encoding four L. (V.) braziliensis antigens: (i) homologue of receptor for activated C kinase, (ii) thiol-specific antioxidant, (iii) Leishmania elongation and initiation factor, and (iv) L. (L.) major stress-inducible protein 1. At the deduced amino acid level, all four open reading frames had a high degree of identity with the previously described genes of L. (L.) major being expressed on promastigotes and amastigotes of L. (V.) braziliensis. These genes were inserted into the vector pcDNA3 or expressed as bacterial recombinant proteins. After immunization with recombinant plasmids or proteins, BALB/c mice generated specific antibody or cell-mediated immune responses (gamma interferon production). After an intradermal challenge with L. (V.) braziliensis infective promastigotes, no significant reduction on the lesions was detected. We conclude that the protective immunity afforded by these four vaccine candidates against experimental cutaneous leishmaniasis caused by L. (L.) major could not be reproduced against a challenge with L. (V.) braziliensis. Although negative, we consider our results important since they suggest that studies aimed at the development of an effective vaccine against L. (V.) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World, should be redirected toward distinct antigens or different vaccination strategies.
Figures


Similar articles
-
Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis.Infect Immun. 2002 Aug;70(8):4215-25. doi: 10.1128/IAI.70.8.4215-4225.2002. Infect Immun. 2002. PMID: 12117930 Free PMC article.
-
Antibody response in patients with cutaneous leishmaniasis infected by Leishmania (Viannia) braziliensis or Leishmania (Viannia) guyanensis in Brazil.Acta Trop. 2005 Jan;93(1):49-56. doi: 10.1016/j.actatropica.2004.09.005. Acta Trop. 2005. PMID: 15589797
-
Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis.Vaccine. 2004 Sep 28;22(29-30):3865-76. doi: 10.1016/j.vaccine.2004.04.015. Vaccine. 2004. PMID: 15364433
-
Challenges and perspectives in vaccination against leishmaniasis.Parasitol Int. 2009 Dec;58(4):319-24. doi: 10.1016/j.parint.2009.07.013. Epub 2009 Aug 19. Parasitol Int. 2009. PMID: 19698801 Review.
-
Leishmaniasis: current status of vaccine development.Curr Mol Med. 2004 Sep;4(6):667-79. doi: 10.2174/1566524043360203. Curr Mol Med. 2004. PMID: 15357215 Review.
Cited by
-
TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia).PLoS Negl Trop Dis. 2011 Jun;5(6):e1204. doi: 10.1371/journal.pntd.0001204. Epub 2011 Jun 14. PLoS Negl Trop Dis. 2011. PMID: 21695103 Free PMC article.
-
Vaccination with L. infantum chagasi nucleosomal histones confers protection against new world cutaneous leishmaniasis caused by Leishmania braziliensis.PLoS One. 2012;7(12):e52296. doi: 10.1371/journal.pone.0052296. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284976 Free PMC article.
-
Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing.Front Cell Infect Microbiol. 2022 Feb 17;11:790418. doi: 10.3389/fcimb.2021.790418. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35252020 Free PMC article.
-
Identification of novel leishmanicidal molecules by virtual and biochemical screenings targeting Leishmania eukaryotic translation initiation factor 4A.PLoS Negl Trop Dis. 2018 Jan 18;12(1):e0006160. doi: 10.1371/journal.pntd.0006160. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29346371 Free PMC article.
-
Coadministration of the Three Antigenic Leishmania infantum Poly (A) Binding Proteins as a DNA Vaccine Induces Protection against Leishmania major Infection in BALB/c Mice.PLoS Negl Trop Dis. 2015 May 8;9(5):e0003751. doi: 10.1371/journal.pntd.0003751. eCollection 2015 May. PLoS Negl Trop Dis. 2015. PMID: 25955652 Free PMC article.
References
-
- Alfieri, S. C., V. Zilberfarb, and M. Rabinovitch. 1987. Destruction of Leishmania mexicana amazonensis amastigotes by leucine methyl ester: protection by other amino acid esters. Parasitology 9531-41. - PubMed
-
- Barbieri, C. L., A. I. Doine, and E. Freymuller. 1990. Lysosomal depletion in macrophages from spleen and foot lesions of Leishmania-infected hamster. Exp. Parasitol. 71218-228. - PubMed
-
- Berman, J. D. 1988. Chemotherapy for leishmaniasis: biochemical-mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 10560-586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

