Inhibition of Pin1 reduces glutamate-induced perikaryal accumulation of phosphorylated neurofilament-H in neurons
- PMID: 17626162
- PMCID: PMC1951754
- DOI: 10.1091/mbc.e07-03-0237
Inhibition of Pin1 reduces glutamate-induced perikaryal accumulation of phosphorylated neurofilament-H in neurons
Abstract
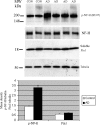
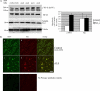
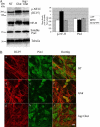
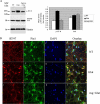
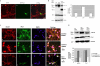
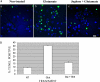
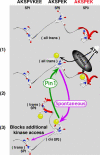
Under normal conditions, the proline-directed serine/threonine residues of neurofilament tail-domain repeats are exclusively phosphorylated in axons. In pathological conditions such as amyotrophic lateral sclerosis (ALS), motor neurons contain abnormal perikaryal accumulations of phosphorylated neurofilament proteins. The precise mechanisms for this compartment-specific phosphorylation of neurofilaments are not completely understood. Although localization of kinases and phosphatases is certainly implicated, another possibility involves Pin1 modulation of phosphorylation of the proline-directed serine/threonine residues. Pin1, a prolyl isomerase, selectively binds to phosphorylated proline-directed serine/threonine residues in target proteins and isomerizes cis isomers to more stable trans configurations. In this study we show that Pin1 associates with phosphorylated neurofilament-H (p-NF-H) in neurons and is colocalized in ALS-affected spinal cord neuronal inclusions. To mimic the pathology of neurodegeneration, we studied glutamate-stressed neurons that displayed increased p-NF-H in perikaryal accumulations that colocalized with Pin1 and led to cell death. Both effects were reduced upon inhibition of Pin1 activity by the use of an inhibitor juglone and down-regulating Pin1 levels through the use of Pin1 small interfering RNA. Thus, isomerization of lys-ser-pro repeat residues that are abundant in NF-H tail domains by Pin1 can regulate NF-H phosphorylation, which suggests that Pin1 inhibition may be an attractive therapeutic target to reduce pathological accumulations of p-NF-H.
Figures

Similar articles
-
Peptidyl-prolyl isomerase 1 regulates protein phosphatase 2A-mediated topographic phosphorylation of neurofilament proteins.J Neurosci. 2009 Nov 25;29(47):14869-80. doi: 10.1523/JNEUROSCI.4469-09.2009. J Neurosci. 2009. PMID: 19940183 Free PMC article.
-
Pin1-dependent prolyl isomerization modulates the stress-induced phosphorylation of high molecular weight neurofilament protein.J Biol Chem. 2008 Sep 26;283(39):26737-47. doi: 10.1074/jbc.M801633200. Epub 2008 Jul 17. J Biol Chem. 2008. PMID: 18635547 Free PMC article.
-
Phosphorylation-specific peptidyl-prolyl isomerization of neuronal cytoskeletal proteins by Pin1: implications for therapeutics in neurodegeneration.J Alzheimers Dis. 2010;19(2):389-403. doi: 10.3233/JAD-2010-1243. J Alzheimers Dis. 2010. PMID: 20110589 Review.
-
Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition.Biochemistry. 1998 Apr 21;37(16):5566-75. doi: 10.1021/bi973060z. Biochemistry. 1998. PMID: 9548941
-
Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and Alzheimer disease.Expert Rev Mol Med. 2011 Jun 20;13:e21. doi: 10.1017/S1462399411001906. Expert Rev Mol Med. 2011. PMID: 21682951 Review.
Cited by
-
Neurofilamentopathy in neurodegenerative diseases.Open Neurol J. 2011;5:58-62. doi: 10.2174/1874205X01105010058. Epub 2011 Aug 26. Open Neurol J. 2011. PMID: 21915226 Free PMC article.
-
The Peptidyl-prolyl Isomerase Pin1 in Neuronal Signaling: from Neurodevelopment to Neurodegeneration.Mol Neurobiol. 2021 Mar;58(3):1062-1073. doi: 10.1007/s12035-020-02179-8. Epub 2020 Oct 21. Mol Neurobiol. 2021. PMID: 33083964 Free PMC article. Review.
-
Effects of Pin1 Loss in Hdh(Q111) Knock-in Mice.Front Cell Neurosci. 2016 May 2;10:110. doi: 10.3389/fncel.2016.00110. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27199664 Free PMC article.
-
The protein level and transcription activity of activating transcription factor 1 is regulated by prolyl isomerase Pin1 in nasopharyngeal carcinoma progression.Cell Death Dis. 2016 Dec 29;7(12):e2571. doi: 10.1038/cddis.2016.349. Cell Death Dis. 2016. PMID: 28032861 Free PMC article.
-
Peptidyl-prolyl isomerase Pin1 markedly enhances the oncogenic activity of the rel proteins in the nuclear factor-kappaB family.Cancer Res. 2009 Jun 1;69(11):4589-97. doi: 10.1158/0008-5472.CAN-08-4117. Epub 2009 May 19. Cancer Res. 2009. PMID: 19458071 Free PMC article.
References
-
- Al-Chalabi A., Miller C. C. Neurofilaments and neurological disease. Bioessays. 2003;25:346–355. - PubMed
-
- Augustinack J. C., Sanders J. L., Tsai L. H., Hyman B. T. Colocalization and fluorescence resonance energy transfer between cdk5 and AT8 suggests a close association in pre-neurofibrillary tangles and neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 2002a;61:557–564. - PubMed
-
- Augustinack J. C., Schneider A., Mandelkow E. M., Hyman B. T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002b;103:26–35. - PubMed
-
- Becker E. B., Bonni A. Pin1 mediates neural-specific activation of the mitochondrial apoptotic machinery. Neuron. 2006;49:655–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

