Gleevec increases levels of the amyloid precursor protein intracellular domain and of the amyloid-beta degrading enzyme neprilysin
- PMID: 17626163
- PMCID: PMC1951756
- DOI: 10.1091/mbc.e07-01-0035
Gleevec increases levels of the amyloid precursor protein intracellular domain and of the amyloid-beta degrading enzyme neprilysin
Abstract
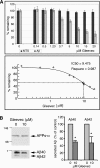
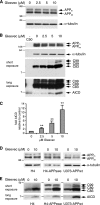
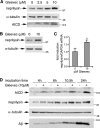
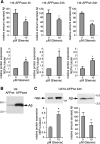
Amyloid-beta (Abeta) deposition is a major pathological hallmark of Alzheimer's disease. Gleevec, a known tyrosine kinase inhibitor, has been shown to lower Abeta secretion, and it is considered a potential basis for novel therapies for Alzheimer's disease. Here, we show that Gleevec decreases Abeta levels without the inhibition of Notch cleavage by a mechanism distinct from gamma-secretase inhibition. Gleevec does not influence gamma-secretase activity in vitro; however, treatment of cell lines leads to a dose-dependent increase in the amyloid precursor protein intracellular domain (AICD), whereas secreted Abeta is decreased. This effect is observed even in presence of a potent gamma-secretase inhibitor, suggesting that Gleevec does not activate AICD generation but instead may slow down AICD turnover. Concomitant with the increase in AICD, Gleevec leads to elevated mRNA and protein levels of the Abeta-degrading enzyme neprilysin, a potential target gene of AICD-regulated transcription. Thus, the Gleevec mediated-increase in neprilysin expression may involve enhanced AICD signaling. The finding that Gleevec elevates neprilysin levels suggests that its Abeta-lowering effect may be caused by increased Abeta-degradation.
Figures



References
-
- Ando K., Iijima K. I., Elliott J. I., Kirino Y., Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J. Biol. Chem. 2001;276:40353–40361. - PubMed
-
- Aplin A. E., Gibb G. M., Jacobsen J. S., Gallo J. M., Anderton B. H. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3beta. J. Neurochem. 1996;67:699–707. - PubMed
-
- Baek S. H., Ohgi K. A., Rose D. W., Koo E. H., Glass C. K., Rosenfeld M. G. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. - PubMed
-
- Bentahir M., Nyabi O., Verhamme J., Tolia A., Horre K., Wiltfang J., Esselmann H., De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J. Neurochem. 2006;96:732–742. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

