Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus
- PMID: 17626164
- PMCID: PMC1951740
- DOI: 10.1091/mbc.e07-01-0025
Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus
Abstract
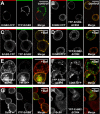
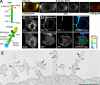
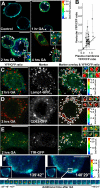
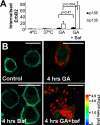
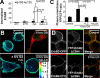
High ErbB2 levels are associated with cancer, and impaired endocytosis of ErbB2 could contribute to its overexpression. Therefore, knowledge about the mechanisms underlying endocytic down-regulation of ErbB2 is warranted. The C-terminus of ErbB2 can be cleaved after various stimuli, and after inhibition of HSP90 with geldanamycin this cleavage is accompanied by proteasome-dependent endocytosis of ErbB2. However, it is unknown whether C-terminal cleavage is linked to endocytosis. To study ErbB2 cleavage and endocytic trafficking, we fused yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) to the N- and C-terminus of ErbB2, respectively (YFP-ErbB2-CFP). After geldanamycin stimulation YFP-ErbB2-CFP became cleaved in nonapoptotic cells in a proteasome-dependent manner, and a markedly larger relative amount of cleaved YFP-ErbB2-CFP was observed in early endosomes than in the plasma membrane. Furthermore, cleavage took place at the plasma membrane, and cleaved ErbB2 was internalized and degraded far more efficiently than full-length ErbB2. Concordantly, a C-terminally truncated ErbB2 was also readily endocytosed and degraded in lysosomes compared with full-length ErbB2. Altogether, we suggest that geldanamycin leads to C-terminal cleavage of ErbB2, which releases the receptor from a retention mechanism and causes endocytosis and lysosomal degradation of ErbB2.
Figures
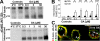

Similar articles
-
Preubiquitinated chimeric ErbB2 is constitutively endocytosed and subsequently degraded in lysosomes.Exp Cell Res. 2013 Feb 1;319(3):32-45. doi: 10.1016/j.yexcr.2012.10.010. Epub 2012 Nov 2. Exp Cell Res. 2013. PMID: 23127513
-
Identification of an HSP90 modulated multi-step process for ERBB2 degradation in breast cancer cells.Oncotarget. 2016 Dec 20;7(51):85411-85429. doi: 10.18632/oncotarget.13392. Oncotarget. 2016. PMID: 27863425 Free PMC article.
-
Geldanamycin stimulates internalization of ErbB2 in a proteasome-dependent way.J Cell Sci. 2006 Jan 1;119(Pt 1):85-95. doi: 10.1242/jcs.02707. Epub 2005 Dec 13. J Cell Sci. 2006. PMID: 16352662
-
Geldanamycin-induced down-regulation of ErbB2 from the plasma membrane is clathrin dependent but proteasomal activity independent.Mol Cancer Res. 2008 Mar;6(3):491-500. doi: 10.1158/1541-7786.MCR-07-0191. Mol Cancer Res. 2008. PMID: 18337455
-
The Mysterious Ways of ErbB2/HER2 Trafficking.Membranes (Basel). 2014 Aug 6;4(3):424-46. doi: 10.3390/membranes4030424. Membranes (Basel). 2014. PMID: 25102001 Free PMC article. Review.
Cited by
-
Chronic morphine treatment attenuates cell growth of human BT474 breast cancer cells by rearrangement of the ErbB signalling network.PLoS One. 2013;8(1):e53510. doi: 10.1371/journal.pone.0053510. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308242 Free PMC article.
-
Expanding the Disorder-Function Paradigm in the C-Terminal Tails of Erbbs.Biomolecules. 2021 Nov 14;11(11):1690. doi: 10.3390/biom11111690. Biomolecules. 2021. PMID: 34827688 Free PMC article. Review.
-
Septin oligomerization regulates persistent expression of ErbB2/HER2 in gastric cancer cells.Biochem J. 2016 Jun 15;473(12):1703-18. doi: 10.1042/BCJ20160203. Epub 2016 Apr 5. Biochem J. 2016. PMID: 27048593 Free PMC article.
-
A combination of two antibodies recognizing non-overlapping epitopes of HER2 induces kinase activity-dependent internalization of HER2.J Cell Mol Med. 2016 Oct;20(10):1999-2011. doi: 10.1111/jcmm.12899. Epub 2016 Jul 28. J Cell Mol Med. 2016. PMID: 27469139 Free PMC article.
-
Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization.Int J Mol Sci. 2016 Jan 14;17(1):102. doi: 10.3390/ijms17010102. Int J Mol Sci. 2016. PMID: 26784176 Free PMC article. Review.
References
-
- Baulida J., Kraus M. H., Alimandi M., Di Fiore P. P., Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 1996;271:5251–5257. - PubMed
-
- Bendtsen J. D., Nielsen H., von H. G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. - PubMed
-
- Burack M. A., Silverman M. A., Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

