Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell
- PMID: 17626201
- PMCID: PMC6672615
- DOI: 10.1523/JNEUROSCI.0710-07.2007
Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell
Abstract
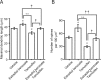
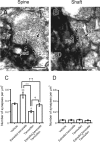
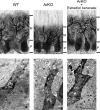
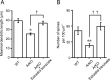
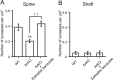
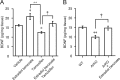
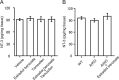
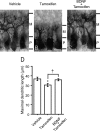
Neurosteroids are synthesized de novo from cholesterol in the brain. To understand neurosteroid action in the brain, data on the regio- and temporal-specific synthesis of neurosteroids are needed. Recently, we identified the Purkinje cell as an active neurosteroidogenic cell. In rodents, this neuron actively produces several neurosteroids including estradiol during neonatal life, when cerebellar neuronal circuit formation occurs. Estradiol may be involved in cerebellar neuronal circuit formation through promoting neuronal growth and neuronal synaptic contact, because the Purkinje cell expresses estrogen receptor-beta (ERbeta). To test this hypothesis, in this study we examined the effects of estradiol on dendritic growth, spinogenesis, and synaptogenesis in the Purkinje cell using neonatal wild-type (WT) mice or cytochrome P450 aromatase knock-out (ArKO) mice. Administration of estradiol to neonatal WT or ArKO mice increased dendritic growth, spinogenesis, and synaptogenesis in the Purkinje cell. In contrast, WT mice treated with tamoxifen, an ER antagonist, or ArKO mice exhibited decreased Purkinje dendritic growth, spinogenesis, and synaptogenesis at the same neonatal period. To elucidate the mode of action of estradiol, we further examined the expression of brain-derived neurotrophic factor (BDNF) in response to estrogen actions in the neonate. Estrogen administration to neonatal WT or ArKO mice increased the BDNF level in the cerebellum, whereas tamoxifen decreased the BDNF level in WT mice similar to ArKO mice. BDNF administration to tamoxifen-treated WT mice increased Purkinje dendritic growth. These results indicate that estradiol induces dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell via BDNF action during neonatal life.
Figures

Similar articles
-
Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF.Cerebellum. 2012 Jun;11(2):416-7. doi: 10.1007/s12311-011-0342-6. Cerebellum. 2012. PMID: 22198873 Review.
-
Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell.Endocrinology. 2003 Oct;144(10):4466-77. doi: 10.1210/en.2003-0307. Epub 2003 Jul 17. Endocrinology. 2003. PMID: 12960093
-
Neurosteroids in the Purkinje cell: biosynthesis, mode of action and functional significance.Mol Neurobiol. 2008 Apr-Jun;37(2-3):116-25. doi: 10.1007/s12035-008-8024-1. Epub 2008 Jun 3. Mol Neurobiol. 2008. PMID: 18521763 Review.
-
Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell.J Steroid Biochem Mol Biol. 2006 Dec;102(1-5):187-94. doi: 10.1016/j.jsbmb.2006.09.015. J Steroid Biochem Mol Biol. 2006. PMID: 17113981 Review.
-
Neurosteroid biosynthesis and action during cerebellar development.Cerebellum. 2012 Jun;11(2):414-5. doi: 10.1007/s12311-011-0341-7. Cerebellum. 2012. PMID: 22198872 Review.
Cited by
-
Gene expression profiling of preplate neurons destined for the subplate: genes involved in transcription, axon extension, neurotransmitter regulation, steroid hormone signaling, and neuronal survival.Cereb Cortex. 2009 Jul;19 Suppl 1(Suppl 1):i126-34. doi: 10.1093/cercor/bhp034. Epub 2009 Apr 27. Cereb Cortex. 2009. PMID: 19398467 Free PMC article.
-
Biosynthesis and biological action of pineal allopregnanolone.Front Cell Neurosci. 2014 May 5;8:118. doi: 10.3389/fncel.2014.00118. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24834027 Free PMC article. Review.
-
Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques.Mol Psychiatry. 2010 Oct;15(10):1034-44. doi: 10.1038/mp.2009.78. Epub 2009 Aug 18. Mol Psychiatry. 2010. PMID: 19687787 Free PMC article.
-
Ketamine attenuates cytochrome p450 aromatase gene expression and estradiol-17β levels in zebrafish early life stages.J Appl Toxicol. 2014 May;34(5):480-8. doi: 10.1002/jat.2888. Epub 2013 May 20. J Appl Toxicol. 2014. PMID: 23696345 Free PMC article.
-
Long-term incubation with mifepristone (MLTI) increases the spine density in developing Purkinje cells: new insights into progesterone receptor mechanisms.Cell Mol Life Sci. 2014 May;71(9):1723-40. doi: 10.1007/s00018-013-1448-4. Epub 2013 Aug 28. Cell Mol Life Sci. 2014. PMID: 23982753 Free PMC article.
References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972a;145:353–398. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972b;145:399–464. - PubMed
-
- Aste N, Honda S, Harada N. Forebrain Fos responses to reproductively related chemosensory cues in aromatase knockout mice. Brain Res Bull. 2003;60:191–200. - PubMed
-
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurosci Res. 1999;58:815–822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
