Lymphotoxin beta receptor (Lt betaR): dual roles in demyelination and remyelination and successful therapeutic intervention using Lt betaR-Ig protein
- PMID: 17626203
- PMCID: PMC6672621
- DOI: 10.1523/JNEUROSCI.1307-07.2007
Lymphotoxin beta receptor (Lt betaR): dual roles in demyelination and remyelination and successful therapeutic intervention using Lt betaR-Ig protein
Abstract
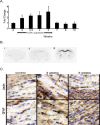
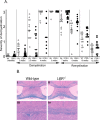
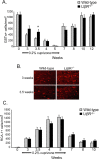
Inflammation mediated by macrophages is increasingly found to play a central role in diseases and disorders that affect a myriad of organs, prominent among these are diseases of the CNS. The neurotoxicant-induced, cuprizone model of demyelination is ideally suited for the analysis of inflammatory events. Demyelination on exposure to cuprizone is accompanied by predictable microglial activation and astrogliosis, and, after cuprizone withdrawal, this activation reproducibly diminishes during remyelination. This study demonstrates enhanced expression of lymphotoxin beta receptor (Lt betaR) during the demyelination phase of this model, and Lt betaR is found in areas enriched with microglial and astroglial cells. Deletion of the Lt betaR gene (Lt betaR-/-) resulted in a significant delay in demyelination but also a slight delay in remyelination. Inhibition of Lt betaR signaling by an Lt betaR-Ig fusion decoy protein successfully delayed demyelination in wild-type mice. Unexpectedly, this Lt betaR-Ig decoy protein dramatically accelerated the rate of remyelination, even after the maximal pathological disease state had been reached. This strongly indicates the beneficial role of Lt betaR-Ig in the delay of demyelination and the acceleration of remyelination. The discrepancy between remyelination rates in these systems could be attributed to developmental abnormalities in the immune systems of Lt betaR-/- mice. These findings bode well for the use of an inhibitory Lt betaR-Ig as a candidate biological therapy in demyelinating disorders, because it is beneficial during both demyelination and remyelination.
Figures
Similar articles
-
Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication.Neurosci Res. 2012 Jan;72(1):32-42. doi: 10.1016/j.neures.2011.10.002. Epub 2011 Oct 12. Neurosci Res. 2012. PMID: 22015947 Free PMC article.
-
Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice.J Neuroimmunol. 1998 Dec 1;92(1-2):38-49. doi: 10.1016/s0165-5728(98)00168-4. J Neuroimmunol. 1998. PMID: 9916878
-
Gene expression in brain during cuprizone-induced demyelination and remyelination.Mol Cell Neurosci. 1998 Nov;12(4-5):220-7. doi: 10.1006/mcne.1998.0715. Mol Cell Neurosci. 1998. PMID: 9828087
-
The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system.Brain Pathol. 2001 Jan;11(1):107-16. doi: 10.1111/j.1750-3639.2001.tb00385.x. Brain Pathol. 2001. PMID: 11145196 Free PMC article. Review.
-
The mechanistic target of rapamycin as a regulator of metabolic function in oligodendroglia during remyelination.Curr Opin Pharmacol. 2022 Apr;63:102193. doi: 10.1016/j.coph.2022.102193. Epub 2022 Mar 1. Curr Opin Pharmacol. 2022. PMID: 35245799 Free PMC article. Review.
Cited by
-
Modeling multiple sclerosis in laboratory animals.Semin Immunopathol. 2009 Nov;31(4):479-95. doi: 10.1007/s00281-009-0181-4. Epub 2009 Oct 3. Semin Immunopathol. 2009. PMID: 19802608 Review.
-
Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned.Front Cell Neurosci. 2014 Mar 13;8:73. doi: 10.3389/fncel.2014.00073. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24659953 Free PMC article. Review.
-
SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients.Lab Invest. 2008 Mar;88(3):243-55. doi: 10.1038/labinvest.3700720. Epub 2008 Jan 21. Lab Invest. 2008. PMID: 18209728 Free PMC article.
-
Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein.J Biotechnol. 2010 Mar;146(1-2):84-91. doi: 10.1016/j.jbiotec.2010.01.011. Epub 2010 Jan 25. J Biotechnol. 2010. PMID: 20100527 Free PMC article.
-
Lymphotoxin β receptor-mediated NFκB signaling promotes glial lineage differentiation and inhibits neuronal lineage differentiation in mouse brain neural stem/progenitor cells.J Neuroinflammation. 2018 Feb 20;15(1):49. doi: 10.1186/s12974-018-1074-z. J Neuroinflammation. 2018. PMID: 29463313 Free PMC article.
References
-
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. - PubMed
-
- Arnett HA, Hellendall RP, Matsushima GK, Suzuki K, Laubach VE, Sherman P, Ting JP. The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. J Immunol. 2002;168:427–433. - PubMed
-
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. - PubMed
-
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
