High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition
- PMID: 17626207
- PMCID: PMC6672616
- DOI: 10.1523/JNEUROSCI.0646-07.2007
High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition
Abstract
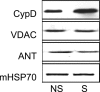
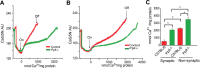
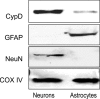
Mitochondria isolated from synaptosomes are more sensitive to Ca2+ overload and the resultant opening of the mitochondrial permeability transition pore (mPTP) than nonsynaptic mitochondria. To identify the mechanisms underlying these differences in Ca2+ dynamics, we examined relative levels of mPTP components in synaptic versus nonsynaptic mitochondria. Synaptic mitochondria had higher levels of cyclophilin D when compared with nonsynaptic mitochondria, whereas levels of the voltage-dependent anion channel and the adenine nucleotide translocase were similar in the two mitochondrial fractions. These differences in Ca2+ handling between synaptic and nonsynaptic mitochondria were greatly reduced in cyclophilin D null [Ppif-/- (peptidylprolyl isomerase F)] mice. Higher concentrations of cyclosporine A, which interacts with cyclophilin D to delay mPTP opening, were necessary to increase the Ca2+ uptake capacity of synaptic versus nonsynaptic mitochondria. To determine whether the differences in Ca2+ handling might reflect the relative abundance of neuronal and glial mitochondria in the two mitochondrial fractions, we compared cyclophilin D levels in primary cortical neurons and astrocytes. Primary rat cortical neurons possess higher cyclophilin D levels than do primary astrocytes. In the adult rat brain, cyclophilin D immunoreactivity was abundant in neurons but sparse in astrocytes. Together, these results demonstrate that the Ca2+ handling differences observed in synaptic versus nonsynaptic mitochondria are primarily the result of the high levels of cyclophilin D in synaptic mitochondria, reflecting the greater proportion of neuronal mitochondria in this fraction. The high levels of cyclophilin D in neuronal mitochondria result in their greater vulnerability to mPT and in higher levels of cyclosporine A being required to inhibit mPTP opening.
Figures


Similar articles
-
Chronic ethanol consumption enhances sensitivity to Ca(2+)-mediated opening of the mitochondrial permeability transition pore and increases cyclophilin D in liver.Am J Physiol Gastrointest Liver Physiol. 2010 Oct;299(4):G954-66. doi: 10.1152/ajpgi.00246.2010. Epub 2010 Jul 22. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20651005 Free PMC article.
-
Phosphate is not an absolute requirement for the inhibitory effects of cyclosporin A or cyclophilin D deletion on mitochondrial permeability transition.Biochem J. 2012 Apr 1;443(1):185-91. doi: 10.1042/BJ20111881. Biochem J. 2012. PMID: 22236255 Free PMC article.
-
Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death.Nature. 2005 Mar 31;434(7033):658-62. doi: 10.1038/nature03434. Nature. 2005. PMID: 15800627
-
Cyclophilin D as a drug target.Curr Med Chem. 2003 Aug;10(16):1485-506. doi: 10.2174/0929867033457160. Curr Med Chem. 2003. PMID: 12871122 Review.
-
Role of the mitochondrial membrane permeability transition in cell death.Apoptosis. 2007 May;12(5):835-40. doi: 10.1007/s10495-006-0525-7. Apoptosis. 2007. PMID: 17136322 Review.
Cited by
-
Microglia-induced neuroinflammation in hippocampal neurogenesis following traumatic brain injury.Heliyon. 2024 Aug 8;10(16):e35869. doi: 10.1016/j.heliyon.2024.e35869. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220913 Free PMC article. Review.
-
Enhancement of anxiety, facilitation of avoidance behavior, and occurrence of adult-onset obesity in mice lacking mitochondrial cyclophilin D.Neuroscience. 2008 Aug 26;155(3):585-96. doi: 10.1016/j.neuroscience.2008.06.030. Epub 2008 Jun 21. Neuroscience. 2008. PMID: 18621101 Free PMC article.
-
Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death.Exp Neurol. 2009 Aug;218(2):171-82. doi: 10.1016/j.expneurol.2009.02.007. Epub 2009 Feb 21. Exp Neurol. 2009. PMID: 19236863 Free PMC article.
-
Signal transduction to the permeability transition pore.FEBS Lett. 2010 May 17;584(10):1989-96. doi: 10.1016/j.febslet.2010.02.022. Epub 2010 Feb 11. FEBS Lett. 2010. PMID: 20153328 Free PMC article. Review.
-
Differential Ca2+ handling by isolated synaptic and non-synaptic mitochondria: roles of Ca2+ buffering and efflux.Front Synaptic Neurosci. 2025 May 27;17:1562065. doi: 10.3389/fnsyn.2025.1562065. eCollection 2025. Front Synaptic Neurosci. 2025. PMID: 40496698 Free PMC article.
References
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
-
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. - PubMed
-
- Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
