High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition
- PMID: 17626207
- PMCID: PMC6672616
- DOI: 10.1523/JNEUROSCI.0646-07.2007
High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition
Abstract
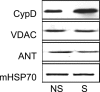
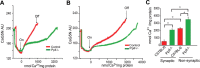
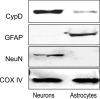
Mitochondria isolated from synaptosomes are more sensitive to Ca2+ overload and the resultant opening of the mitochondrial permeability transition pore (mPTP) than nonsynaptic mitochondria. To identify the mechanisms underlying these differences in Ca2+ dynamics, we examined relative levels of mPTP components in synaptic versus nonsynaptic mitochondria. Synaptic mitochondria had higher levels of cyclophilin D when compared with nonsynaptic mitochondria, whereas levels of the voltage-dependent anion channel and the adenine nucleotide translocase were similar in the two mitochondrial fractions. These differences in Ca2+ handling between synaptic and nonsynaptic mitochondria were greatly reduced in cyclophilin D null [Ppif-/- (peptidylprolyl isomerase F)] mice. Higher concentrations of cyclosporine A, which interacts with cyclophilin D to delay mPTP opening, were necessary to increase the Ca2+ uptake capacity of synaptic versus nonsynaptic mitochondria. To determine whether the differences in Ca2+ handling might reflect the relative abundance of neuronal and glial mitochondria in the two mitochondrial fractions, we compared cyclophilin D levels in primary cortical neurons and astrocytes. Primary rat cortical neurons possess higher cyclophilin D levels than do primary astrocytes. In the adult rat brain, cyclophilin D immunoreactivity was abundant in neurons but sparse in astrocytes. Together, these results demonstrate that the Ca2+ handling differences observed in synaptic versus nonsynaptic mitochondria are primarily the result of the high levels of cyclophilin D in synaptic mitochondria, reflecting the greater proportion of neuronal mitochondria in this fraction. The high levels of cyclophilin D in neuronal mitochondria result in their greater vulnerability to mPT and in higher levels of cyclosporine A being required to inhibit mPTP opening.
Figures


References
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
-
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. - PubMed
-
- Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
