Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation
- PMID: 17626212
- PMCID: PMC6672605
- DOI: 10.1523/JNEUROSCI.0705-07.2007
Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation
Abstract
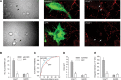
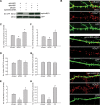
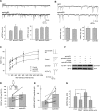
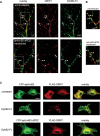
Excitatory synapses in the CNS are formed on both dendritic spines and shafts. Recent studies show that the density of shaft synapses may be independently regulated by behavioral learning and the induction of synaptic plasticity, suggesting that distinct mechanisms are involved in regulating these two types of synapses. Although the molecular mechanisms underlying spinogenesis and spine synapse formation are being delineated, those regulating shaft synapses are still unknown. Here, we show that postsynaptic ephrinB3 expression promotes the formation of glutamatergic synapses specifically on the shafts, not on spines. Reducing or increasing postsynaptic ephrinB3 expression selectively decreases or increases shaft synapse density, respectively. In the ephrinB3 knock-out mouse, although spine synapses are normal, shaft synapse formation is reduced in the hippocampus. Overexpression of glutamate receptor-interacting protein 1 (GRIP1) rescues ephrinB3 knockdown phenotype by restoring shaft synapse density. GRIP1 knockdown prevents the increase in shaft synapse density induced by ephrinB3 overexpression. Together, our results reveal a novel mechanism for independent modulation of shaft synapses through ephrinB3 reverse signaling.
Figures



Similar articles
-
Distinct roles for ephrinB3 in the formation and function of hippocampal synapses.Dev Biol. 2006 Apr 1;292(1):34-45. doi: 10.1016/j.ydbio.2006.01.004. Epub 2006 Feb 8. Dev Biol. 2006. PMID: 16466709
-
Synaptic modifications at the CA3-CA1 synapse after chronic AMPA receptor blockade in rat hippocampal slices.J Physiol. 2007 May 15;581(Pt 1):129-38. doi: 10.1113/jphysiol.2006.120550. Epub 2007 Feb 15. J Physiol. 2007. PMID: 17303644 Free PMC article.
-
Persistence of excitatory shaft synapses adjacent to newly emerged dendritic protrusions.Mol Cell Neurosci. 2011 Oct;48(2):129-36. doi: 10.1016/j.mcn.2011.06.014. Epub 2011 Jul 13. Mol Cell Neurosci. 2011. PMID: 21784157 Free PMC article.
-
Imaging of spine synapses using super-resolution microscopy.Anat Sci Int. 2021 Jun;96(3):343-358. doi: 10.1007/s12565-021-00603-0. Epub 2021 Jan 18. Anat Sci Int. 2021. PMID: 33459976 Review.
-
Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways.Curr Opin Neurobiol. 2010 Feb;20(1):108-15. doi: 10.1016/j.conb.2009.09.013. Epub 2009 Nov 4. Curr Opin Neurobiol. 2010. PMID: 19896363 Free PMC article. Review.
Cited by
-
EphA signaling promotes actin-based dendritic spine remodeling through slingshot phosphatase.J Biol Chem. 2012 Mar 16;287(12):9346-59. doi: 10.1074/jbc.M111.302802. Epub 2012 Jan 26. J Biol Chem. 2012. PMID: 22282498 Free PMC article.
-
AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking.Cell Mol Life Sci. 2019 Jun;76(11):2133-2169. doi: 10.1007/s00018-019-03068-7. Epub 2019 Apr 1. Cell Mol Life Sci. 2019. PMID: 30937469 Free PMC article. Review.
-
Synaptic cell adhesion.Cold Spring Harb Perspect Biol. 2012 Apr 1;4(4):a005694. doi: 10.1101/cshperspect.a005694. Cold Spring Harb Perspect Biol. 2012. PMID: 22278667 Free PMC article.
-
Presenilin1/gamma-secretase promotes the EphB2-induced phosphorylation of ephrinB2 by regulating phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk binding protein.FASEB J. 2011 Oct;25(10):3594-604. doi: 10.1096/fj.11-187856. Epub 2011 Jul 11. FASEB J. 2011. PMID: 21746865 Free PMC article.
-
EphrinBs send mixed messages.Nat Neurosci. 2011 Oct 26;14(11):1356-8. doi: 10.1038/nn.2968. Nat Neurosci. 2011. PMID: 22030542 No abstract available.
References
-
- Anderson JC, Martin KA. Synaptic connection from cortical area V4 to V2 in macaque monkey. J Comp Neurol. 2006;495:709–721. - PubMed
-
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
-
- Bozhilova-Pastirova A, Ovtscharoff W. Structure of the synaptic junctions in the rat sensorimotor cortex. Freeze-etching study of axodendritic synapses. Eur J Morphol. 1996;34:363–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
