An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex
- PMID: 17626216
- PMCID: PMC6672607
- DOI: 10.1523/JNEUROSCI.1786-07.2007
An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex
Abstract
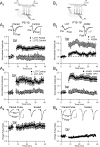
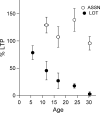
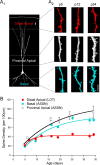
Critical periods for plasticity of thalamic sensory inputs play an important role in developing neocortical circuits. During an early postnatal time window, pyramidal cells of visual, auditory, and somatosensory cortex undergo structural refinement and possess an enhanced ability for activity-dependent synaptic plasticity. In olfactory cortex, however, pyramidal cells receive direct sensory input from the olfactory bulb, and it is unclear whether the development of olfactory sensory circuits is governed by a critical period. Here, we show that NMDA receptor-dependent long-term potentiation and dendritic spine maturation occur only during a brief postnatal time window at sensory synapses of olfactory cortex pyramidal cells. In contrast, associational synapses onto the same cells retain the capacity for plasticity into adulthood.
Figures
References
-
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. - PubMed
-
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. - PubMed
-
- Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Ed 4. New York: Oxford UP; 1998. pp. 377–416.
-
- Haberly LB, Presto S. Ultrastructural analysis of synaptic relationships of intracellularly stained pyramidal cell axons in piriform cortex. J Comp Neurol. 1986;248:464–474. - PubMed
-
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
