Corticortophin releasing factor 2 receptor agonist treatment significantly slows disease progression in mdx mice
- PMID: 17626629
- PMCID: PMC1936998
- DOI: 10.1186/1741-7015-5-18
Corticortophin releasing factor 2 receptor agonist treatment significantly slows disease progression in mdx mice
Abstract
Background: Duchenne muscular dystrophy results from mutation of the dystrophin gene, causing skeletal and cardiac muscle loss of function. The mdx mouse model of Duchenne muscular dystrophy is widely utilized to evaluate the potential of therapeutic regimens to modulate the loss of skeletal muscle function associated with dystrophin mutation. Importantly, progressive loss of diaphragm function is the most consistent striated muscle effect observed in the mdx mouse model, which is the same as in patients suffering from Duchenne muscular dystrophy.
Methods: Using the mdx mouse model, we have evaluated the effect that corticotrophin releasing factor 2 receptor (CRF2R) agonist treatment has on diaphragm function, morphology and gene expression.
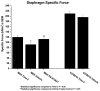
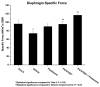
Results: We have observed that treatment with the potent CRF2R-selective agonist PG-873637 prevents the progressive loss of diaphragm specific force observed during aging of mdx mice. In addition, the combination of PG-873637 with glucocorticoids not only prevents the loss of diaphragm specific force over time, but also results in recovery of specific force. Pathological analysis of CRF2R agonist-treated diaphragm muscle demonstrates that treatment reduces fibrosis, immune cell infiltration, and muscle architectural disruption. Gene expression analysis of CRF2R-treated diaphragm muscle showed multiple gene expression changes including globally decreased immune cell-related gene expression, decreased extracellular matrix gene expression, increased metabolism-related gene expression, and, surprisingly, modulation of circadian rhythm gene expression.
Conclusion: Together, these data demonstrate that CRF2R activation can prevent the progressive degeneration of diaphragm muscle associated with dystrophin gene mutation.
Figures

Similar articles
-
Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy.Hum Mol Genet. 2004 Feb 1;13(3):257-69. doi: 10.1093/hmg/ddh033. Epub 2003 Dec 17. Hum Mol Genet. 2004. PMID: 14681298
-
Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers.Hum Mol Genet. 2011 May 1;20(9):1787-99. doi: 10.1093/hmg/ddr062. Epub 2011 Feb 14. Hum Mol Genet. 2011. PMID: 21320869
-
[Dystrophin expression and pathology of diaphragm muscles of mdx mice after xenogenic bone marrow stem cell transplantation].Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):53-8. Nan Fang Yi Ke Da Xue Xue Bao. 2006. PMID: 16495176 Chinese.
-
An attempt of gene therapy in Duchenne muscular dystrophy: overexpression of utrophin in transgenic mdx mice.Acta Neurol Belg. 2000 Sep;100(3):146-50. Acta Neurol Belg. 2000. PMID: 11098286 Review.
-
Diaphragm muscle strip preparation for evaluation of gene therapies in mdx mice.Clin Exp Pharmacol Physiol. 2008 Jul;35(7):725-9. doi: 10.1111/j.1440-1681.2007.04865.x. Epub 2008 Jan 21. Clin Exp Pharmacol Physiol. 2008. PMID: 18215182 Review.
Cited by
-
Treatment with a corticotrophin releasing factor 2 receptor agonist modulates skeletal muscle mass and force production in aged and chronically ill animals.BMC Musculoskelet Disord. 2011 Jan 14;12:15. doi: 10.1186/1471-2474-12-15. BMC Musculoskelet Disord. 2011. PMID: 21235761 Free PMC article.
-
Anabolic and Pro-metabolic Functions of CREB-CRTC in Skeletal Muscle: Advantages and Obstacles for Type 2 Diabetes and Cancer Cachexia.Front Endocrinol (Lausanne). 2019 Aug 2;10:535. doi: 10.3389/fendo.2019.00535. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31428057 Free PMC article. Review.
-
cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration.Am J Physiol Endocrinol Metab. 2012 Jul 1;303(1):E1-17. doi: 10.1152/ajpendo.00555.2011. Epub 2012 Feb 21. Am J Physiol Endocrinol Metab. 2012. PMID: 22354781 Free PMC article. Review.
-
Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy.Dis Model Mech. 2014 Jan;7(1):41-54. doi: 10.1242/dmm.013631. Epub 2013 Sep 25. Dis Model Mech. 2014. PMID: 24077965 Free PMC article.
-
Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy.J Pathol. 2012 Sep;228(1):77-87. doi: 10.1002/path.4054. Epub 2012 Jul 18. J Pathol. 2012. PMID: 22653783 Free PMC article.
References
-
- Engel AG, Yamamoto M, Fischbeck KH. Dystrophinopathies. In: Engel AG, Franzini-Armstrong C, editor. Myology. Columbus, OH: McGraw-Hill; 1994. pp. 1133–1187.
-
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

