Overexpressed TP73 induces apoptosis in medulloblastoma
- PMID: 17626635
- PMCID: PMC1955450
- DOI: 10.1186/1471-2407-7-127
Overexpressed TP73 induces apoptosis in medulloblastoma
Abstract
Background: Medulloblastoma is the most common malignant brain tumor of childhood. Children who relapse usually die of their disease, which reflects resistance to radiation and/or chemotherapy. Improvements in outcome require a better understanding of the molecular basis of medulloblastoma growth and treatment response. TP73 is a member of the TP53 tumor suppressor gene family that has been found to be overexpressed in a variety of tumors and mediates apoptotic responses to genotoxic stress. In this study, we assessed expression of TP73 RNA species in patient tumor specimens and in medulloblastoma cell lines, and manipulated expression of full-length TAp73 and amino-terminal truncated DeltaNp73 to assess their effects on growth.
Methods: We analyzed medulloblastoma samples from thirty-four pediatric patients and the established medulloblastoma cell lines, Daoy and D283MED, for expression of TP73 RNA including the full-length transcript and the 5'-terminal variants that encode the DeltaNp73 isoform, as well as TP53 RNA using quantitative real time-RTPCR. Protein expression of TAp73 and DeltaNp73 was quantitated with immunoblotting methods. Clinical outcome was analyzed based on TP73 RNA and p53 protein expression. To determine effects of overexpression or knock-down of TAp73 and DeltaNp73 on cell cycle and apoptosis, we analyzed transiently transfected medulloblastoma cell lines with flow cytometric and TUNEL methods.
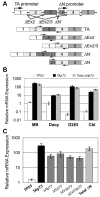
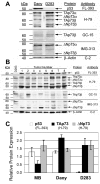
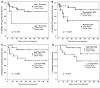
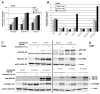
Results: Patient medulloblastoma samples and cell lines expressed full-length and 5'-terminal variant TP73 RNA species in 100-fold excess compared to non-neoplastic brain controls. Western immunoblot analysis confirmed their elevated levels of TAp73 and amino-terminal truncated DeltaNp73 proteins. Kaplan-Meier analysis revealed trends toward favorable overall and progression-free survival of patients whose tumors display TAp73 RNA overexpression. Overexpression of TAp73 or DeltaNp73 induced apoptosis under basal growth conditions in vitro and sensitized them to cell death in response to chemotherapeutic agents.
Conclusion: These results indicate that primary medulloblastomas express significant levels of TP73 isoforms, and suggest that they can modulate the survival and genotoxic responsiveness of medulloblastomas cells.
Figures

Similar articles
-
p73 expression in medulloblastoma: TAp73/DeltaNp73 transcript detection and possible association of p73alpha/DeltaNp73 immunoreactivity with survival.Acta Neuropathol. 2007 Dec;114(6):641-50. doi: 10.1007/s00401-007-0298-2. Epub 2007 Oct 3. Acta Neuropathol. 2007. PMID: 17912537
-
EZH2-regulated DAB2IP is a medulloblastoma tumor suppressor and a positive marker for survival.Clin Cancer Res. 2012 Aug 1;18(15):4048-58. doi: 10.1158/1078-0432.CCR-12-0399. Epub 2012 Jun 13. Clin Cancer Res. 2012. PMID: 22696229
-
DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors.J Exp Med. 2002 Sep 16;196(6):765-80. doi: 10.1084/jem.20020179. J Exp Med. 2002. PMID: 12235210 Free PMC article.
-
TP73, an under-appreciated player in non-Hodgkin lymphoma pathogenesis and management.Curr Mol Med. 2014 May;14(4):432-9. doi: 10.2174/1566524014666140414204458. Curr Mol Med. 2014. PMID: 24730526 Review.
-
Clinical relevance of TAp73 and ΔNp73 protein expression in ovarian cancer: a series of 83 cases and review of the literature.Int J Gynecol Pathol. 2011 Nov;30(6):527-31. doi: 10.1097/PGP.0b013e31821ac519. Int J Gynecol Pathol. 2011. PMID: 21979586 Review.
Cited by
-
NPI-0052 and γ-radiation induce a synergistic apoptotic effect in medulloblastoma.Cell Death Dis. 2019 Oct 16;10(11):785. doi: 10.1038/s41419-019-2026-y. Cell Death Dis. 2019. PMID: 31619667 Free PMC article.
-
Overexpression of the ∆Np73 isoform is associated with centrosome amplification in brain tumor cell lines.Tumour Biol. 2015 Sep;36(10):7483-91. doi: 10.1007/s13277-015-3474-3. Epub 2015 Apr 25. Tumour Biol. 2015. PMID: 25910708
-
WIP1 modulates responsiveness to Sonic Hedgehog signaling in neuronal precursor cells and medulloblastoma.Oncogene. 2016 Oct 20;35(42):5552-5564. doi: 10.1038/onc.2016.96. Epub 2016 Apr 18. Oncogene. 2016. PMID: 27086929 Free PMC article.
-
Cross-talk between T-Ag presence and pRb family and p53/p73 signaling in mouse and human medulloblastoma.J Cell Biochem. 2010 May;110(1):182-90. doi: 10.1002/jcb.22525. J Cell Biochem. 2010. PMID: 20336668 Free PMC article.
-
TAp73 represses NF-κB-mediated recruitment of tumor-associated macrophages in breast cancer.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2017089118. doi: 10.1073/pnas.2017089118. Proc Natl Acad Sci U S A. 2021. PMID: 33649219 Free PMC article.
References
-
- Giangaspero F, Bigner SH, Giordana MT, Kleihues P, Trojanowski JQ. Medulloblastoma. In: Kleihues P, Cavenee WK, editor. Pathology and Genetics: Tumours of the Nervous System World Health Organization Classification of Tumours. Lyon, France: International Agency for Research of Cancer; 2000. pp. 96–103.
-
- Ohgaki H, Eibl RH, Wiestler OD, Yasargil MG, Newcomb EW, Kleihues P. p53 mutations in nonastrocytic human brain tumors. Cancer Res. 1991;51:6202–6205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

