Regulation of protein kinase CK1alphaLS by dephosphorylation in response to hydrogen peroxide
- PMID: 17626781
- PMCID: PMC2131699
- DOI: 10.1016/j.abb.2007.06.010
Regulation of protein kinase CK1alphaLS by dephosphorylation in response to hydrogen peroxide
Abstract
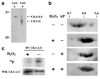
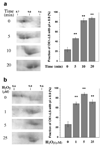
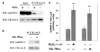
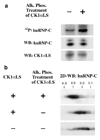
Low levels of hydrogen peroxide (H(2)O(2)) are mitogenic to mammalian cells and stimulate the hyperphosphorylation of heterogeneous nuclear ribonucleoprotein C (hnRNP-C) by protein kinase CK1alpha. However, the mechanisms by which CK1alpha is regulated have been unclear. Here it is demonstrated that low levels of H(2)O(2) stimulate the rapid dephosphorylation of CK1alphaLS, a nuclear splice form of CK1alpha. Furthermore, it is demonstrated that either treatment of endothelial cells with H(2)O(2), or dephosphorylation of CK1alphaLS in vitro enhances the association of CK1alphaLS with hnRNP-C. In addition, dephosphorylation of CK1alphaLS in vitro enhances the kinase's ability to phosphorylate hnRNP-C. While CK1alpha appears to be present in all metazoans, analysis of CK1alpha genomic sequences from several species reveals that the alternatively spliced nuclear localizing L-insert is unique to vertebrates, as is the case for hnRNP-C. These observations indicate that CK1alphaLS and hnRNP-C represent conserved components of a vertebrate-specific H(2)O(2)-responsive nuclear signaling pathway.
Figures

Similar articles
-
Protein kinase CK1alphaLS promotes vascular cell proliferation and intimal hyperplasia.Am J Pathol. 2010 Sep;177(3):1562-72. doi: 10.2353/ajpath.2010.100327. Epub 2010 Aug 9. Am J Pathol. 2010. PMID: 20696773 Free PMC article.
-
Protein kinase CK1alpha regulates mRNA binding by heterogeneous nuclear ribonucleoprotein C in response to physiologic levels of hydrogen peroxide.J Biol Chem. 2005 Apr 15;280(15):15340-7. doi: 10.1074/jbc.M500214200. Epub 2005 Feb 1. J Biol Chem. 2005. PMID: 15687492
-
Altered vascular activation due to deficiency of the NADPH oxidase component p22phox.Cardiovasc Pathol. 2014 Jan-Feb;23(1):35-42. doi: 10.1016/j.carpath.2013.08.003. Epub 2013 Sep 12. Cardiovasc Pathol. 2014. PMID: 24035466
-
Rapid phosphorylation of heterogeneous nuclear ribonucleoprotein C1/C2 in response to physiologic levels of hydrogen peroxide in human endothelial cells.J Biol Chem. 2002 May 3;277(18):15621-8. doi: 10.1074/jbc.M112153200. Epub 2002 Feb 27. J Biol Chem. 2002. PMID: 11877401
-
Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction.Exp Biol Med (Maywood). 2006 Mar;231(3):237-51. doi: 10.1177/153537020623100302. Exp Biol Med (Maywood). 2006. PMID: 16514169 Review.
Cited by
-
Protein kinase CK1alphaLS promotes vascular cell proliferation and intimal hyperplasia.Am J Pathol. 2010 Sep;177(3):1562-72. doi: 10.2353/ajpath.2010.100327. Epub 2010 Aug 9. Am J Pathol. 2010. PMID: 20696773 Free PMC article.
-
Fuzzy interactions between the auto-phosphorylated C-terminus and the kinase domain of CK1δ inhibits activation of TAp63α.Sci Rep. 2023 Sep 30;13(1):16423. doi: 10.1038/s41598-023-43515-x. Sci Rep. 2023. PMID: 37777570 Free PMC article.
-
Hydrogen peroxide sensing, signaling and regulation of transcription factors.Redox Biol. 2014 Feb 23;2:535-62. doi: 10.1016/j.redox.2014.02.006. eCollection 2014. Redox Biol. 2014. PMID: 24634836 Free PMC article. Review.
-
The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis.Front Oncol. 2014 May 19;4:96. doi: 10.3389/fonc.2014.00096. eCollection 2014. Front Oncol. 2014. PMID: 24904820 Free PMC article. Review.
-
Exploiting the MDM2-CK1α protein-protein interface to develop novel biologics that induce UBL-kinase-modification and inhibit cell growth.PLoS One. 2012;7(8):e43391. doi: 10.1371/journal.pone.0043391. Epub 2012 Aug 20. PLoS One. 2012. PMID: 22916255 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

