Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells
- PMID: 17626796
- PMCID: PMC5239667
- DOI: 10.1124/jpet.107.124545
Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells
Abstract
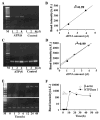
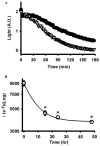
Stimulation of receptors for either ATP or adenosine leads to physiologic changes in retinal pigment epithelial (RPE) cells that may influence their relationship with the adjacent photoreceptors. The ectoenzyme nucleoside-triphosphate diphosphohydrolase-1 (NTPDase1) catalyzes the dual dephosphorylation of ATP and ADP to AMP. Although NTPDase1 can consequently control the balance between ATP and adenosine, it is unclear how its expression and activity are regulated. Classic negative feedback theory predicts an increase in enzyme activity in response to enhanced exposure to substrate. This study asked whether exposure to ATP increases NTPDase1 activity in RPE cells. Although levels of NTPDase1 mRNA and protein in cultured human ARPE-19 cells were generally low under control conditions, exposure to slowly hydrolyzable ATPgammaS led to a time-dependent increase in NTPDase1 mRNA that was accompanied by a rise in levels of the functional 78-kDa protein. Neither NTPDase2 nor NTPDase3 mRNA message was elevated by ATPgammaS. The ATPase activity of cells increased in parallel, indicating the up-regulation of NTPDase1 was functionally relevant. The up-regulation of NTPDase1 protein was partially blocked by P2Y1 receptor inhibitors MRS2179 (N6-methyl-2'-deoxyadenosine-3',5'-bisphosphate) and MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine 3',5'-bisphosphate] and increased by P2Y1 receptor agonist MRS2365 [(N)-methanocarba-2MeSADP]. In conclusion, prolonged exposure to extracellular ATPgammaS increased NTPDase1 message and protein levels and increased ecto-ATPase activity. This up-regulation reflects a feedback circuit, mediated at least in part by the P2Y1 receptor, to regulate levels of extracellular purines in subretinal space. NTPDase1 levels may thus serve as an index for increased extracellular ATP levels under certain pathologic conditions, although other mechanisms could also contribute.
Figures



Similar articles
-
Degradation of extracellular ATP by the retinal pigment epithelium.Am J Physiol Cell Physiol. 2005 Sep;289(3):C617-24. doi: 10.1152/ajpcell.00542.2004. Epub 2005 Apr 27. Am J Physiol Cell Physiol. 2005. PMID: 15857904
-
Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2.Mol Pharmacol. 2005 Jan;67(1):114-22. doi: 10.1124/mol.104.006908. Epub 2004 Oct 20. Mol Pharmacol. 2005. PMID: 15496502
-
The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation.Br J Pharmacol. 2010 Feb 1;159(3):576-85. doi: 10.1111/j.1476-5381.2009.00566.x. Epub 2010 Jan 8. Br J Pharmacol. 2010. PMID: 20067476 Free PMC article.
-
A fusion protein of the human P2Y(1) receptor and NTPDase1 exhibits functional activities of the native receptor and ectoenzyme and reduced signaling responses to endogenously released nucleotides.Mol Pharmacol. 2002 Sep;62(3):521-8. doi: 10.1124/mol.62.3.521. Mol Pharmacol. 2002. PMID: 12181428
-
Pharmacological profiles of cloned mammalian P2Y-receptor subtypes.Pharmacol Ther. 2006 Jun;110(3):415-32. doi: 10.1016/j.pharmthera.2005.08.014. Epub 2005 Oct 28. Pharmacol Ther. 2006. PMID: 16257449 Review.
Cited by
-
Targeting the P2X7 Receptor in Age-Related Macular Degeneration.Vision (Basel). 2017 Mar 31;1(2):11. doi: 10.3390/vision1020011. Vision (Basel). 2017. PMID: 31740637 Free PMC article. Review.
-
Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain.Glia. 2014 Sep;62(9):1486-501. doi: 10.1002/glia.22695. Epub 2014 May 19. Glia. 2014. PMID: 24839011 Free PMC article.
-
Intraocular kinetics of pathological ATP after photoreceptor damage in rhegmatogenous retinal detachment.Jpn J Ophthalmol. 2024 Sep;68(5):500-510. doi: 10.1007/s10384-024-01087-x. Epub 2024 Jul 26. Jpn J Ophthalmol. 2024. PMID: 39060674
-
Nicotinic Acetylcholine Receptor Partial Antagonist Polyamides from Tunicates and Their Predatory Sea Slugs.ACS Chem Neurosci. 2021 Jul 21;12(14):2693-2704. doi: 10.1021/acschemneuro.1c00345. Epub 2021 Jul 2. ACS Chem Neurosci. 2021. PMID: 34213884 Free PMC article.
-
Increased Purinergic Signaling in Human Dental Pulps With Inflammatory Pain is Sex-Dependent.J Pain. 2024 Apr;25(4):1039-1058. doi: 10.1016/j.jpain.2023.10.026. Epub 2023 Nov 11. J Pain. 2024. PMID: 37956743 Free PMC article.
References
-
- Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. - PubMed
-
- Baurand A, Gachet C. The P2Y1 receptor as a target for new antithrombotic drugs: a review of the P2Y1 antagonist MRS-2179. Cardiovasc Drug Rev. 2003;21:67–76. - PubMed
-
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. - PubMed
-
- Bonan CD, Walz R, Pereira GS, Worm PV, Battastini AM, Cavalheiro EA, Izquierdo I, Sarkis JJ. Changes in synaptosomal ectonucleotidase activities in two rat models of temporal lobe epilepsy. Epilepsy Res. 2000;39:229–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

