Natural killer cells trigger differentiation of monocytes into dendritic cells
- PMID: 17626840
- PMCID: PMC1988958
- DOI: 10.1182/blood-2007-02-076364
Natural killer cells trigger differentiation of monocytes into dendritic cells
Abstract
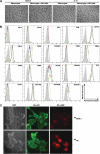
Circulating monocytes can differentiate into dendritic cells (moDCs), which are potent inducers of adaptive immune responses. Previous reports show that granulocyte macrophage-colony-stimulating factor (GM-CSF) and interleukin-4 induce monocyte differentiation into moDCs in vitro, but little is known about the physiological requirements that initiate moDC differentiation in vivo. Here we show that a unique natural killer (NK) cell subset (CD3(-)CD56(bright)) that accumulates in lymph nodes and chronically inflamed tissues triggers CD14(+) monocytes to differentiate into potent T-helper-1 (T(H)1) promoting DC. This process requires direct contact of monocytes with NK cells and is mediated by GM-CSF and CD154 derived from NK cells. It is noteworthy that synovial fluid (SF) from patients with rheumatoid arthritis (RA) and psoriatic arthritis (PsA), but not osteoarthritis (OA), induces monocytes to differentiate into DC. However, this process occurs only in the presence of NK cells. We propose that NK cells play a role in the maintenance of T(H)1-mediated inflammatory diseases such as RA by providing a local milieu for monocytes to differentiate into DC.
Figures
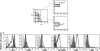




Similar articles
-
Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis.Ann Rheum Dis. 2016 May;75(5):899-907. doi: 10.1136/annrheumdis-2014-206578. Epub 2015 Apr 28. Ann Rheum Dis. 2016. PMID: 25923217 Free PMC article.
-
NK cells enhance the induction of CTL responses by IL-15 monocyte-derived dendritic cells.Immunol Cell Biol. 2009 Nov-Dec;87(8):606-14. doi: 10.1038/icb.2009.44. Epub 2009 Jun 23. Immunol Cell Biol. 2009. PMID: 19546878
-
A novel role for IL-3: human monocytes cultured in the presence of IL-3 and IL-4 differentiate into dendritic cells that produce less IL-12 and shift Th cell responses toward a Th2 cytokine pattern.J Immunol. 2002 Jun 15;168(12):6199-207. doi: 10.4049/jimmunol.168.12.6199. J Immunol. 2002. PMID: 12055233
-
Shaping of Monocyte-Derived Dendritic Cell Development and Function by Environmental Factors in Rheumatoid Arthritis.Int J Mol Sci. 2021 Dec 20;22(24):13670. doi: 10.3390/ijms222413670. Int J Mol Sci. 2021. PMID: 34948462 Free PMC article. Review.
-
Mechanisms of NK cell activation: CD4(+) T cells enter the scene.Cell Mol Life Sci. 2011 Nov;68(21):3457-67. doi: 10.1007/s00018-011-0796-1. Epub 2011 Aug 23. Cell Mol Life Sci. 2011. PMID: 21861183 Free PMC article. Review.
Cited by
-
Natural killer cells in human autoimmune disorders.Arthritis Res Ther. 2013 Jul 11;15(4):216. doi: 10.1186/ar4232. Arthritis Res Ther. 2013. PMID: 23856014 Free PMC article. Review.
-
The Pleiotropic Effects of the GM-CSF Rheostat on Myeloid Cell Differentiation and Function: More Than a Numbers Game.Front Immunol. 2019 Nov 15;10:2679. doi: 10.3389/fimmu.2019.02679. eCollection 2019. Front Immunol. 2019. PMID: 31803190 Free PMC article. Review.
-
Natural killer cells in human autoimmune diseases.Immunology. 2010 Dec;131(4):451-8. doi: 10.1111/j.1365-2567.2010.03360.x. Epub 2010 Oct 13. Immunology. 2010. PMID: 21039469 Free PMC article. Review.
-
The Interplay between the bone and the immune system.Clin Dev Immunol. 2013;2013:720504. doi: 10.1155/2013/720504. Epub 2013 Jul 14. Clin Dev Immunol. 2013. PMID: 23935650 Free PMC article. Review.
-
Arthritis models: usefulness and interpretation.Semin Immunopathol. 2017 Jun;39(4):469-486. doi: 10.1007/s00281-017-0622-4. Epub 2017 Mar 27. Semin Immunopathol. 2017. PMID: 28349194 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

