Orexins control intestinal glucose transport by distinct neuronal, endocrine, and direct epithelial pathways
- PMID: 17626888
- PMCID: PMC2214858
- DOI: 10.2337/db07-0614
Orexins control intestinal glucose transport by distinct neuronal, endocrine, and direct epithelial pathways
Abstract
Objective: Orexins are neuropeptides involved in energy homeostasis. We investigated the effect of orexin A (OxA) and orexin B (OxB) on intestinal glucose transport in the rat.
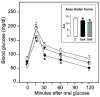
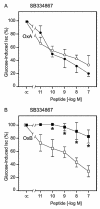
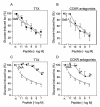
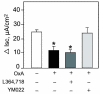
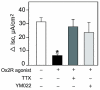
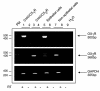
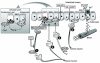
Research design and methods and results: Injection of orexins led to a decrease in the blood glucose level in oral glucose tolerance tests (OGTTs). Effects of orexins on glucose entry were analyzed in Ussing chambers using the Na(+)-dependent increase in short-circuit current (Isc) to quantify jejunal glucose transport. The rapid and marked increase in Isc induced by luminal glucose was inhibited by 10 nmol/l OxA or OxB (53 and 59%, respectively). Response curves to OxA and OxB were not significantly different with half-maximal inhibitory concentrations at 0.9 and 0.4 nmol/l, respectively. On the one hand, OxA-induced inhibition of Isc was reduced by the neuronal blocker tetrodotoxin (TTX) and by a cholecystokinin (CCK) 2R antagonist, indicating involvement of neuronal and endocrine CCK-releasing cells. The OX(1)R antagonist SB334867 had no effect on OxA-induced inhibition, which is likely to occur via a neuronal and/or endocrine OX(2)R. On the other hand, SB334867 induced a significant right shift of the concentration-effect curve for OxB. This OxB-preferring OX(1)R pathway was not sensitive to TTX or to CCKR antagonists, suggesting that OxB may act directly on enterocytic OX(1)R. These distinct effects of OxA and OxB are consistent with the expression of OX(1)R and OX(2)R mRNA in the epithelial and nonepithelial tissues, respectively.
Conclusions: Our data delineate a new function for orexins as inhibitors of intestinal glucose absorption and provide a new basis for orexin-induced short-term control of energy homeostasis.
Figures

References
-
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. - PubMed
-
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. - PMC - PubMed
-
- Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951. - PubMed
-
- Ehrstrom M, Gustafsson T, Finn A, Kirchgessner A, Gryback P, Jacobsson H, Hellstrom PM, Naslund E. Inhibitory effect of exogenous orexin a on gastric emptying, plasma leptin, and the distribution of orexin and orexin receptors in the gut and pancreas in man. J Clin Endocrinol Metab. 2005;90:2370–2377. - PubMed

