The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation
- PMID: 17627302
- PMCID: PMC1906732
- DOI: 10.1172/JCI31024
The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation
Abstract
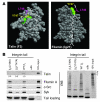
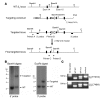
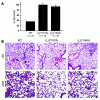
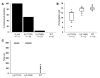
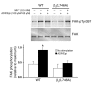
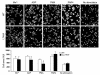
In vitro studies indicate that binding of talin to the beta(3) integrin cytoplasmic domain (tail) results in integrin alpha(IIb)beta(3) (GPIIb-IIIa) activation. Here we tested the importance of talin binding for integrin activation in vivo and its biological significance by generating mice harboring point mutations in the beta(3) tail. We introduced a beta(3)(Y747A) substitution that disrupts the binding of talin, filamin, and other cytoplasmic proteins and a beta(3)(L746A) substitution that selectively disrupts interactions only with talin. Platelets from animals homozygous for each mutation showed impaired agonist-induced fibrinogen binding and platelet aggregation, providing proof that inside-out signals that activate alpha(IIb)beta(3) require binding of talin to the beta(3) tail. beta(3)(L746A) mice were resistant to both pulmonary thromboembolism and to ferric chloride-induced thrombosis of the carotid artery. Pathological bleeding, measured by the presence of fecal blood and development of anemia, occurred in 53% of beta(3)(Y747A) and virtually all beta(3)-null animals examined. Remarkably, less than 5% of beta(3)(L746A) animals exhibited this form of bleeding. These results establish that alpha(IIb)beta(3) activation in vivo is dependent on the interaction of talin with the beta(3) integrin cytoplasmic domain. Furthermore, they suggest that modulation of beta(3) integrin-talin interactions may provide an attractive target for antithrombotics and result in a reduced risk of pathological bleeding.
Figures

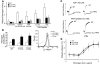
References
-
- Quinn M.J., Byzova T.V., Qin J., Topol E.J., Plow E.F. Integrin alphaIIbbeta3 and its antagonism. Arterioscler. Thromb. Vasc. Biol. 2003;23:945–952. - PubMed
-
- Chew D.P., Bhatt D.L., Sapp S., Topol E.J. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. - PubMed
-
- Shattil S.J., Newman P.J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. - PubMed
-
- Ginsberg M.H., Partridge A., Shattil S.J. Integrin regulation. Curr. Opin. Cell. Biol. 2005;17:509–516. - PubMed
-
- Bhatt D.L., Topol E.J. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2003;2:15–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

